HSV-1 DNA polymerase 3'-5' exonuclease-deficient mutant D368A exhibits severely reduced viral DNA synthesis and polymerase expression
- PMID: 30176164
- PMCID: PMC7011708
- DOI: 10.1099/jgv.0.001138
HSV-1 DNA polymerase 3'-5' exonuclease-deficient mutant D368A exhibits severely reduced viral DNA synthesis and polymerase expression
Abstract
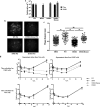
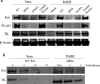
Herpesviruses, including herpes simplex virus-1, encode and express a DNA polymerase that is required for replication of their dsDNA genomes. The catalytic subunit of this enzyme contains a 3'-5' exonuclease that is involved in proofreading during replication. Although certain mutations that severely impair exonuclease activity are not lethal to the virus, it was reported that virus containing the substitution of alanine for aspartate 368 (D368A), which ablates exonuclease activity, could not be recovered, raising the possibility that this activity is essential for viral replication. To investigate this issue, we produced virus containing this mutation (D368A Pol) using a complementing cell line. D368A Pol virus was unable to form plaques on non-complementing cells. Viral DNA synthesis and polymerase activity were severely inhibited in D368A-infected cells, as was expression of the enzyme, suggesting that effects on polymerase expression rather than on exonuclease activity per se largely explain the lethal phenotype of this mutation.
Keywords: 3’-5’ exonuclease; DNA polymerase; DNA replication; Herpes smplex virus-1.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

Similar articles
-
Herpes Simplex Virus 1 DNA Polymerase RNase H Activity Acts in a 3'-to-5' Direction and Is Dependent on the 3'-to-5' Exonuclease Active Site.J Virol. 2018 Feb 12;92(5):e01813-17. doi: 10.1128/JVI.01813-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237844 Free PMC article.
-
Roles of conserved residues within the pre-NH2-terminal domain of herpes simplex virus 1 DNA polymerase in replication and latency in mice.J Gen Virol. 2014 Apr;95(Pt 4):940-947. doi: 10.1099/vir.0.061903-0. Epub 2014 Jan 10. J Gen Virol. 2014. PMID: 24413420 Free PMC article.
-
Finger domain mutation affects enzyme activity, DNA replication efficiency, and fidelity of an exonuclease-deficient DNA polymerase of herpes simplex virus type 1.J Virol. 2009 Jul;83(14):7194-201. doi: 10.1128/JVI.00632-09. Epub 2009 May 6. J Virol. 2009. PMID: 19420083 Free PMC article.
-
Distribution and effects of amino acid changes in drug-resistant α and β herpesviruses DNA polymerase.Nucleic Acids Res. 2016 Nov 16;44(20):9530-9554. doi: 10.1093/nar/gkw875. Epub 2016 Sep 29. Nucleic Acids Res. 2016. PMID: 27694307 Free PMC article. Review.
-
The structure and function of the HSV DNA replication proteins: defining novel antiviral targets.Antiviral Res. 1993 Feb;20(2):89-114. doi: 10.1016/0166-3542(93)90001-y. Antiviral Res. 1993. PMID: 8384825 Review.
Cited by
-
A proofreading-impaired herpesvirus generates populations with quasispecies-like structure.Nat Microbiol. 2019 Dec;4(12):2175-2183. doi: 10.1038/s41564-019-0547-x. Epub 2019 Sep 2. Nat Microbiol. 2019. PMID: 31477893
-
Psoromic Acid, a Lichen-Derived Molecule, Inhibits the Replication of HSV-1 and HSV-2, and Inactivates HSV-1 DNA Polymerase: Shedding Light on Antiherpetic Properties.Molecules. 2019 Aug 11;24(16):2912. doi: 10.3390/molecules24162912. Molecules. 2019. PMID: 31405197 Free PMC article.
-
Mechanisms of Coronavirus Genome Stability As Potential Targets for Antiviral Drugs.Her Russ Acad Sci. 2022;92(4):470-478. doi: 10.1134/S1019331622040256. Epub 2022 Sep 6. Her Russ Acad Sci. 2022. PMID: 36091852 Free PMC article.
-
Two-Metal Ion-Dependent Enzymes as Potential Antiviral Targets in Human Herpesviruses.mBio. 2022 Feb 22;13(1):e0322621. doi: 10.1128/mbio.03226-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073739 Free PMC article.
-
Suicidal Phenotype of Proofreading-Deficient Herpes Simplex Virus 1 Polymerase Mutants.J Virol. 2023 Jan 31;97(1):e0135922. doi: 10.1128/jvi.01359-22. Epub 2023 Jan 4. J Virol. 2023. PMID: 36598203 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

