Management of patients with hereditary angioedema in Germany: comparison with other countries in the Icatibant Outcome Survey
- PMID: 30176179
- PMCID: PMC6587717
- DOI: 10.1111/jdv.15232
Management of patients with hereditary angioedema in Germany: comparison with other countries in the Icatibant Outcome Survey
Abstract
Background: The Icatibant Outcome Survey (IOS; NCT01034969) is a Shire-sponsored, international, observational study monitoring the safety and effectiveness of icatibant, a bradykinin B2 receptor antagonist approved for the acute treatment of adults with hereditary angioedema with C1 inhibitor deficiency (HAE-C1-INH).
Objective: To report IOS data comparing demographic and icatibant treatment outcomes in patients with HAE-C1-INH from Germany to HAE-C1-INH patients from 11 other IOS countries.
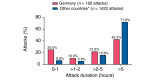
Methods: A descriptive, retrospective, comparative analysis of data from 685 IOS patients with HAE-C1-INH from seven centres in Germany (n = 93) vs. centres from Austria, Brazil, Czech Republic, Denmark, France, Greece, Israel, Italy, Spain, Sweden and the United Kingdom (n = 592, July 2009-January 2017). Icatibant treatment outcomes were retrieved from patients with complete attack outcome data for time to treatment, time to resolution and attack duration (160 attacks in 42 German patients and 1442 attacks in 251 patients from other IOS countries).
Results: German patients reported significantly fewer severe/very severe attacks (38.7% vs. 57.5%, respectively; P < 0.001). The proportion of attacks treated with a single icatibant injection was significantly higher in German patients (97.1% vs. 91.6%, P = 0.0003). The median time to treatment (0.0 h vs. 1.5 h), time to resolution (3.0 h vs. 7.0 h) and attack duration (4.3 h vs. 10.5 h) in German patients vs. other IOS countries were all significantly shorter (all P < 0.0001). No meaningful differences were identified between patients from Germany and other countries with regard to sex, median age at enrolment, median age at symptom onset and median age at diagnosis.
Conclusion: German IOS patients share similar demographic characteristics to patients from other IOS countries yet treat their attacks with icatibant significantly earlier and have markedly fewer severe or very severe attacks. Factors including regional access to and availability of icatibant may drive these outcomes and warrant further investigation.
© 2018 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures



References
-
- Cicardi M, Aberer W, Banerji A et al Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy 2014; 69: 602–616. - PubMed
-
- Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 2006; 119: 267–274. - PubMed
-
- Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1‐INH deficiency. J Allergy Clin Immunol 2012; 130: 692–697. - PubMed
-
- Maurer M, Magerl M, Ansotegui I et al The international WAO/EAACI guideline for the management of hereditary angioedema‐The 2017 revision and update. Allergy 2018; 73: 1575–1596. - PubMed
-
- Jose J, Zacharias J, Craig T. Review of select practice parameters, evidence‐based treatment algorithms, and international guidelines for hereditary angioedema. Clin Rev Allergy Immunol 2016; 51: 193–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

