Stress Adaptation Upregulates Oxytocin within Hypothalamo-Vagal Neurocircuits
- PMID: 30176320
- PMCID: PMC6192260
- DOI: 10.1016/j.neuroscience.2018.08.021
Stress Adaptation Upregulates Oxytocin within Hypothalamo-Vagal Neurocircuits
Abstract
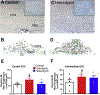
Stress plays a pivotal role in the development and/or exacerbation of functional gastrointestinal (GI) disorders. The paraventricular nucleus of the hypothalamus (PVN) contains neurons that are part of the hypothalamic-pituitary-adrenal axis as well as preautonomic neurons innervating, among other areas, gastric-projecting preganglionic neurons of the dorsal vagal complex (DVC). The aim of the present study was to test the hypothesis that stress adaptation upregulates oxytocin (OXT) within PVN-brainstem vagal neurocircuitry. The retrograde tracer cholera toxin B (CTB) was injected into the DVC of rats which, after post-surgical recovery, were pair-housed and exposed to either homo- or heterotypic stress for five consecutive days. Fecal pellets were counted at the end of each stress load. Two hours after the last stressor, the whole brain was excised. Brainstem and hypothalamic nuclei were analyzed immunohistochemically for the presence of both OXT-immunopositive cells in identified preautonomic PVN neurons as well as OXT fibers in the DVC. Rats exposed to chronic homotypic, but not chronic heterotypic stress, had a significant increase in both number of CTB+ OXT co-localized neurons in the PVN as well as density of OXT-positive fibers in the DVC compared to control rats. These data suggest that preautonomic OXT PVN neurons and their projections to the DVC increase following adaptation to stress, and suggest that the possible up-regulation of OXT within PVN-brainstem vagal neurocircuitry may play a role in the adaptation of GI responses to stress.
Keywords: brainstem; gastrointestinal motility; vagus.
Copyright © 2018 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
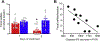

Similar articles
-
Hypothalamic-vagal oxytocinergic neurocircuitry modulates gastric emptying and motility following stress.J Physiol. 2020 Nov;598(21):4941-4955. doi: 10.1113/JP280023. Epub 2020 Sep 7. J Physiol. 2020. PMID: 32864736 Free PMC article.
-
Phenotypic traits of the hypothalamic PVN cells innervating airway-related vagal preganglionic neurons.Respir Physiol Neurobiol. 2006 Dec;154(3):319-30. doi: 10.1016/j.resp.2006.01.006. Epub 2006 Mar 3. Respir Physiol Neurobiol. 2006. PMID: 16515895 Free PMC article.
-
Perinatal high-fat diet exposure alters oxytocin and corticotropin releasing factor inputs onto vagal neurocircuits controlling gastric motility.J Physiol. 2023 Jul;601(14):2853-2875. doi: 10.1113/JP284726. Epub 2023 May 17. J Physiol. 2023. PMID: 37154244 Free PMC article.
-
Fluorescent visualization of oxytocin in the hypothalamo-neurohypophysial system.Front Neurosci. 2014 Jul 23;8:213. doi: 10.3389/fnins.2014.00213. eCollection 2014. Front Neurosci. 2014. PMID: 25100939 Free PMC article. Review.
-
Oxytocin in growth, reproduction, restoration and health.Compr Psychoneuroendocrinol. 2024 Sep 30;20:100268. doi: 10.1016/j.cpnec.2024.100268. eCollection 2024 Nov. Compr Psychoneuroendocrinol. 2024. PMID: 39435014 Free PMC article. Review.
Cited by
-
Stress-induced modulation of vagal afferents.Neurogastroenterol Motil. 2019 Dec;31(12):e13758. doi: 10.1111/nmo.13758. Neurogastroenterol Motil. 2019. PMID: 31736236 Free PMC article. Review.
-
Functional Status of Hypothalamic-Pituitary-Thyroid and Hypothalamic-Pituitary-Adrenal Axes in Hospitalized Schizophrenics in Shanghai.Front Psychiatry. 2020 Feb 27;11:65. doi: 10.3389/fpsyt.2020.00065. eCollection 2020. Front Psychiatry. 2020. PMID: 32174848 Free PMC article.
-
Vagus nerve damage increases alcohol intake and preference in a nonpreferring rat line: Relationship to vagal regulation of the hypothalamic-pituitary-adrenal axis.Alcohol Clin Exp Res (Hoboken). 2024 Mar;48(3):488-498. doi: 10.1111/acer.15264. Epub 2024 Feb 4. Alcohol Clin Exp Res (Hoboken). 2024. PMID: 38311347 Free PMC article.
-
Stress-induced neuroplasticity in the gastric response to brainstem oxytocin in male rats.Am J Physiol Gastrointest Liver Physiol. 2022 May 1;322(5):G513-G522. doi: 10.1152/ajpgi.00347.2021. Epub 2022 Feb 16. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 35170350 Free PMC article.
-
Hypothalamic-vagal oxytocinergic neurocircuitry modulates gastric emptying and motility following stress.J Physiol. 2020 Nov;598(21):4941-4955. doi: 10.1113/JP280023. Epub 2020 Sep 7. J Physiol. 2020. PMID: 32864736 Free PMC article.
References
-
- Altschuler SM, Bao X, Bieger D, Hopkins DA & Miselis RR (1989) Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J. Comp. Neurol, 283, 248–268. - PubMed
-
- Andresen MC & Kunze DL (1994) Nucleus tractus solitarius--gateway to neural circulatory control. Annu. Rev. Physiol, 56, 93–116. - PubMed
-
- Babygirija R, Bulbul M, Cerjak D, Ludwig K & Takahashi T (2011) Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci. Lett, 495, 77–81. - PubMed
-
- Babygirija R, Bulbul M, Yoshimoto S, Ludwig K & Takahashi T (2012) Central and peripheral release of oxytocin following chronic homotypic stress in rats. Auton. Neurosci, 167, 56–60. - PubMed
-
- Babygirija R, Zheng J, Bulbul M, Cerjak D, Ludwig K & Takahashi T (2010a) Sustained delayed gastric emptying during repeated restraint stress in oxytocin knockout mice. J. Neuroendocrinol, 22, 1181–1186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

