Aberrant Glycosylation of the IgA1 Molecule in IgA Nephropathy
- PMID: 30177018
- PMCID: PMC7170174
- DOI: 10.1016/j.semnephrol.2018.05.016
Aberrant Glycosylation of the IgA1 Molecule in IgA Nephropathy
Abstract
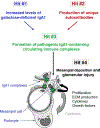
IgA nephropathy, the most common primary glomerulonephritis in the world and a frequent cause of end-stage renal disease, is characterized by typical mesangial deposits of IgA1, as described by Berger and Hinglaise in 1968. Since then, it has been discovered that aberrant IgA1 O-glycosylation is involved in disease pathogenesis. Progress in glycomic, genomic, clinical, analytical, and biochemical studies has shown autoimmune features of IgA nephropathy. The autoimmune character of the disease is explained by a multihit pathogenesis model, wherein overproduction of aberrantly glycosylated IgA1, galactose-deficient in some O-glycans, by IgA1-secreting cells leads to increased levels of circulatory galactose-deficient IgA1. These glycoforms induce production of autoantibodies that subsequently bind hinge-region of galactose-deficient IgA1 molecules, resulting in the formation of nephritogenic immune complexes. Some of these complexes deposit in the kidney, activate mesangial cells, and incite glomerular injury. Thus, galactose-deficient IgA1 is central to the disease process. In this article, we review studies concerning IgA1 O-glycosylation that have contributed to the current understanding of the role of IgA1 in the pathogenesis of IgA nephropathy.
Keywords: IgA1; O-glycans; autoantibody; signaling.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures

References
-
- Julian BA, Waldo FB, Rifai A, Mestecky J. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am J Med. 1988;84:129–32. - PubMed
-
- Berger J, Hinglais N. Les dépôts intercapillaires d’IgA-IgG (Intercapillary deposits of IgA-IgG). J Urol Nephrol. 1968;74:694–5. - PubMed
-
- Jennette JC. The immunohistology of IgA nephropathy. Am J Kidney Dis. 1988;12(5):348–52. - PubMed
-
- Allen AC, Bailey EM, Brenchley PEC, Buck KS, Barratt J, Feehally J. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: Observations in three patients. Kidney Int. 2001;60:969–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

