Randomized phase II trial of bevacizumab plus everolimus versus bevacizumab alone for recurrent or persistent ovarian, fallopian tube or peritoneal carcinoma: An NRG oncology/gynecologic oncology group study
- PMID: 30177462
- PMCID: PMC6350932
- DOI: 10.1016/j.ygyno.2018.08.027
Randomized phase II trial of bevacizumab plus everolimus versus bevacizumab alone for recurrent or persistent ovarian, fallopian tube or peritoneal carcinoma: An NRG oncology/gynecologic oncology group study
Abstract
Purpose: Bevacizumab (BV) monotherapy leads to compensatory upregulation of multiple signaling pathways, resulting in mTOR activation. We evaluated combining BV and everolimus (EV), an mTOR kinase inhibitor, to circumvent BV-resistance in women with recurrent or persistent ovarian, fallopian tube or primary peritoneal cancer (OC).
Patients and methods: Eligible OC patients had measurable (RECIST1.1) or detectable disease, 1-3 prior regimens, performance status (PS) 0-2, and no prior m-TOR inhibitor. All patients received BV 10 mg/kg IV every 2wks. Patients were randomized (1:1) to oral EV (10 mg daily) or placebo stratified by platinum-free interval (PFI), measurable disease and prior BV. Primary endpoint was progression-free survival (PFS); secondary endpoints included safety and response.
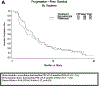
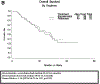
Results: 150 patients were randomized to BV with (n = 75) and without (n = 75) EV. Arms were well-balanced for age (median 63: range 28-92), PS (0: 73%, 1-2: 27%), prior regimens (1: 37%, 2: 47%, 3: 16%), prior BV (11%), PFI (<6mos: 65%) and measurable disease (81%). The BV + EV vs BV median PFS was 5.9 vs 4.5 months (hazard ratio [HR] 0.95 (95% CI, 0.66-1.37, p = 0.39)). Median OS was 16.6 vs 17.3 months (HR 1.16 (95% CI, 0.72-1.87, p = 0.55). Objective measurable responses were higher with BV + EV (22% vs 12%). Study removal due to toxicity was higher with BV + EV (29% vs 12%). Toxicity (≥grade 3) from BV + EV were "other GI (mucositis)" (23 vs 1%) and "metabolic/nutrition" (19 vs. 7%); common ≥ grade 2 toxicities with BV + EV were cytopenia, nausea, fatigue and rash.
Conclusion: The combination regimen (BV + EV) did not significantly reduce the hazard of progression or death relative to BV and was associated with higher rates of adverse events and study discontinuation when compared to BV alone.
Keywords: Bevacizumab; Everolimus; Ovarian cancer; Targeted therapy.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
Dr. Angeles Secord reports grants from NCTN Grant and Gynecologic Oncology Group Grant during the conduct of the study; grants from Astra Zeneca, Eisai, Bristol Myers Squibb, Incyte, Amgen, Genentech, Endocyte, Exelixis, Boerhinger Ingelheim, Astex Pharmaceuticals Inc., Prima Biomed, Abbie-Vie, Astellas Pharma Inc., Tesaro, PharmaMar, Merck, other from Janssen, Clovis, Genentech, Astra Zeneca, Astex, Tesaro, Alexion, Boerhinger Ingelheim, Myriad, Arivave, outside the submitted work.
Dr. Jeanne Schilder received a grant from the NRG/GOG.
Dr. Krishnansu Tewari received grant monies from Genentech for Clinical Trial at UC Irvine. He also received payment for lectures including service on speakers bureaus for CME activities only from Genentech/Roche.
Dr. Carol Aghajanian received money paid to her for consultancy from Tesaro, Mateon Therapeutics, Clovis, Cerulean, Bayer and VentriRx.
All other co-authors had no conflicts of interest to declare.
Figures
References
-
- Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol 1999; 6(4):373–378. - PubMed
-
- Chen CA, Cheng WF, Lee CN, Chen TM, Kung CC, Hsieh FJ, et al. Serum VEGF in epithelial ovarian neoplasms: correlation with patient survival. Gynecol Oncol 1999; 74(2):235–240. - PubMed
-
- Burger RA, Sill M, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007. November 20; 25(33):5165–5171. - PubMed
-
- Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol 2007. November 20; 25(33):5180–5186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous