Modulation of the immune response by helminths: a role for serotonin?
- PMID: 30177522
- PMCID: PMC6148219
- DOI: 10.1042/BSR20180027
Modulation of the immune response by helminths: a role for serotonin?
Abstract
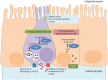
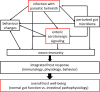
The mammalian gut is a remarkable organ: with a nervous system that rivals the spinal cord, it is the body's largest repository of immune and endocrine cells and houses an immense and complex microbiota. Infection with helminth parasites elicits a conserved program of effector and regulatory immune responses to eradicate the worm, limit tissue damage, and return the gut to homeostasis. Discrete changes in the nervous system, and to a lesser extent the enteroendocrine system, occur following helminth infection but the importance of these adaptations in expelling the worm is poorly understood. Approximately 90% of the body's serotonin (5-hydroxytryptamine (5-HT)) is made in enterochromaffin (EC) cells in the gut, indicative of the importance of this amine in intestinal function. Signaling via a plethora of receptor subtypes, substantial evidence illustrates that 5-HT affects immunity. A small number of studies document changes in 5-HT levels following infection with helminth parasites, but these have not been complemented by an understanding of the role of 5-HT in the host-parasite interaction. In reviewing this area, the gap in knowledge of how changes in the enteric serotonergic system affects the outcome of infection with intestinal helminths is apparent. We present this as a call-to-action by investigators in the field. We contend that neuronal EC cell-immune interactions in the gut are essential in maintaining homeostasis and, when perturbed, contribute to pathophysiology. The full affect of infection with helminth parasites needs to define, and then mechanistically dissect the role of the enteric nervous and enteroendocrine systems of the gut.
Keywords: enterochromaffin cell; helminth; intestine; serotonin.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
The ever-changing roles of serotonin.Int J Biochem Cell Biol. 2020 Aug;125:105776. doi: 10.1016/j.biocel.2020.105776. Epub 2020 May 29. Int J Biochem Cell Biol. 2020. PMID: 32479926 Review.
-
The role of serotonin and its receptors in activation of immune responses and inflammation.Acta Physiol (Oxf). 2015 Mar;213(3):561-74. doi: 10.1111/apha.12430. Epub 2014 Dec 11. Acta Physiol (Oxf). 2015. PMID: 25439045 Review.
-
TLR2 Plays a Pivotal Role in Mediating Mucosal Serotonin Production in the Gut.J Immunol. 2019 May 15;202(10):3041-3052. doi: 10.4049/jimmunol.1801034. Epub 2019 Apr 5. J Immunol. 2019. PMID: 30952815
-
Diverse Effects of Gut-Derived Serotonin in Intestinal Inflammation.ACS Chem Neurosci. 2017 May 17;8(5):920-931. doi: 10.1021/acschemneuro.6b00414. Epub 2017 Mar 27. ACS Chem Neurosci. 2017. PMID: 28288510 Review.
-
The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine.Int J Mol Sci. 2021 Jul 25;22(15):7931. doi: 10.3390/ijms22157931. Int J Mol Sci. 2021. PMID: 34360695 Free PMC article. Review.
Cited by
-
Enteric Microbiota-Mediated Serotonergic Signaling in Pathogenesis of Irritable Bowel Syndrome.Int J Mol Sci. 2021 Sep 23;22(19):10235. doi: 10.3390/ijms221910235. Int J Mol Sci. 2021. PMID: 34638577 Free PMC article. Review.
-
Interleukin-33 Induces the Enzyme Tryptophan Hydroxylase 1 to Promote Inflammatory Group 2 Innate Lymphoid Cell-Mediated Immunity.Immunity. 2020 Apr 14;52(4):606-619.e6. doi: 10.1016/j.immuni.2020.02.009. Epub 2020 Mar 10. Immunity. 2020. PMID: 32160524 Free PMC article.
-
The Role of the Intestinal Epithelium in the "Weep and Sweep" Response during Gastro-Intestinal Helminth Infections.Animals (Basel). 2022 Jan 12;12(2):175. doi: 10.3390/ani12020175. Animals (Basel). 2022. PMID: 35049796 Free PMC article. Review.
-
Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases.Front Immunol. 2020 Feb 11;11:186. doi: 10.3389/fimmu.2020.00186. eCollection 2020. Front Immunol. 2020. PMID: 32117308 Free PMC article. Review.
-
The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective.Front Immunol. 2023 Oct 9;14:1277102. doi: 10.3389/fimmu.2023.1277102. eCollection 2023. Front Immunol. 2023. PMID: 37876938 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

