Silicon Dioxide Nanoparticles Enhance Endotoxin-Induced Lung Injury in Mice
- PMID: 30177658
- PMCID: PMC6225156
- DOI: 10.3390/molecules23092247
Silicon Dioxide Nanoparticles Enhance Endotoxin-Induced Lung Injury in Mice
Abstract
Silicon dioxide nanoparticles (SiONPs), which are metal oxide nanoparticles, have been used in a wide variety of applications. In this study, acute pulmonary responses were examined after the intranasal instillation of SiONPs in mice primed with or without lipopolysaccharide (LPS, intranasal, 5 µg/mouse). The exposure to SiONPs increased the inflammatory cell counts and proinflammatory cytokines in the bronchoalveolar lavage fluid. SiONPs induced airway inflammation with increases in the phosphorylation of mitogen-activated protein kinases (MAPKs). The ratios of the inflammatory responses induced by the SiONPs were increased in the acute pulmonary disease model primed by LPS. Taken together, SiONPs exhibited toxicity to the respiratory system, which was associated with MAPK phosphorylation. In addition, the exposure to SiONPs exacerbated any existing inflammatory pulmonary diseases. These data showed the additive, as well as synergistic, interaction effects of SiONPs and LPS. We conclude that the exposure to SiONPs causes potential toxicity in humans, especially those with respiratory diseases.
Keywords: inflammation; mitogen-activated protein kinase; respiratory tract; silicon dioxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

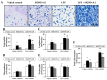

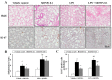

References
-
- Ko J.W., Park J.W., Shin N.R., Kim J.H., Cho Y.K., Shin D.H., Kim J.C., Lee I.C., Oh S.R., Ahn K.S., et al. Copper oxide nanoparticle induces inflammatory response and mucus production via MAPK signaling in human bronchial epithelial cells. Environ. Toxicol. Pharmacol. 2016;43:21–26. doi: 10.1016/j.etap.2016.02.008. - DOI - PubMed
-
- Ahmad J., Ahamed M., Akhtar M.J., Alrokayan S.A., Siddiqui M.A., Musarrat J., Al-Khedhairy A.A. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol. Appl. Pharmacol. 2012;259:160–168. doi: 10.1016/j.taap.2011.12.020. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

