Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles
- PMID: 30177691
- PMCID: PMC6120897
- DOI: 10.1038/s41467-018-06020-8
Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles
Abstract
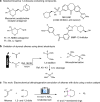
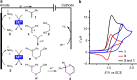
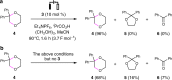
Dehydrogenative annulation reactions are among the most straightforward and efficient approach for the preparation of cyclic structures. However, the applications of this strategy for the synthesis of saturated heterocycles have been rare. In addition, reported dehydrogenative bond-forming reactions commonly employ stoichiometric chemical oxidants, the use of which reduces the sustainability of the synthesis and brings safety and environmental issues. Herein, we report an organocatalyzed electrochemical dehydrogenative annulation reaction of alkenes with 1,2- and 1,3-diols for the synthesis of 1,4-dioxane and 1,4-dioxepane derivatives. The combination of electrochemistry and redox catalysis using an organic catalyst allows the electrosynthesis to proceed under transition metal- and oxidizing reagent-free conditions. In addition, the electrolytic method has a broad substrate scope and is compatible with many common functional groups, providing an efficient and straightforward access to functionalized 1,4-dioxane and 1,4-dioxepane products with diverse substitution patterns.
Conflict of interest statement
The authors declare no competing interests.
Figures
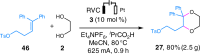
Similar articles
-
Synthesis of N-Heterocycles by Dehydrogenative Annulation of N-Allyl Amides with 1,3-Dicarbonyl Compounds.Angew Chem Int Ed Engl. 2018 Oct 22;57(43):14070-14074. doi: 10.1002/anie.201807683. Epub 2018 Oct 2. Angew Chem Int Ed Engl. 2018. PMID: 30184314
-
External oxidant-free electrooxidative [3 + 2] annulation between phenol and indole derivatives.Nat Commun. 2017 Oct 3;8(1):775. doi: 10.1038/s41467-017-00873-1. Nat Commun. 2017. PMID: 28974679 Free PMC article.
-
Photoelectrocatalytic [4+2] Annulation for S-Heterocycle Assembly Enabled by Proton-Coupled Electron Transfer (PCET).Chemistry. 2024 Oct 23;30(59):e202402333. doi: 10.1002/chem.202402333. Epub 2024 Oct 7. Chemistry. 2024. PMID: 39096120
-
Recent advances in transition metal-catalyzed (1,n) annulation using (de)-hydrogenative coupling with alcohols.Chem Commun (Camb). 2021 Sep 28;57(77):9807-9819. doi: 10.1039/d1cc03404a. Chem Commun (Camb). 2021. PMID: 34486592 Review.
-
Metal-Free Cross-Dehydrogenative Coupling (CDC): Molecular Iodine as a Versatile Catalyst/Reagent for CDC Reactions.Chem Asian J. 2019 Jan 4;14(1):6-30. doi: 10.1002/asia.201801237. Epub 2018 Nov 2. Chem Asian J. 2019. PMID: 30259704 Review.
Cited by
-
Synthetic Molecular Photoelectrochemistry: New Frontiers in Synthetic Applications, Mechanistic Insights and Scalability.Angew Chem Int Ed Engl. 2022 Mar 14;61(12):e202107811. doi: 10.1002/anie.202107811. Epub 2022 Feb 9. Angew Chem Int Ed Engl. 2022. PMID: 34478188 Free PMC article. Review.
-
Redox-active molecules as organocatalysts for selective oxidative transformations - an unperceived organocatalysis field.Beilstein J Org Chem. 2022 Dec 9;18:1672-1695. doi: 10.3762/bjoc.18.179. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 36570566 Free PMC article.
-
Nickel-catalyzed switchable asymmetric electrochemical functionalization of alkenes.Sci Adv. 2022 Nov 11;8(45):eadd7134. doi: 10.1126/sciadv.add7134. Epub 2022 Nov 9. Sci Adv. 2022. PMID: 36351023 Free PMC article.
-
Practical and stereoselective electrocatalytic 1,2-diamination of alkenes.Nat Commun. 2019 Oct 31;10(1):4953. doi: 10.1038/s41467-019-13024-5. Nat Commun. 2019. PMID: 31672991 Free PMC article.
-
Electrochemical C-C bond cleavage of cyclopropanes towards the synthesis of 1,3-difunctionalized molecules.Nat Commun. 2021 May 24;12(1):3075. doi: 10.1038/s41467-021-23401-8. Nat Commun. 2021. PMID: 34031421 Free PMC article.
References
-
- Lovering F. Escape from flatland 2: complexity and promiscuity. Med. Chem. Commun. 2013;4:515–519. doi: 10.1039/c2md20347b. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

