Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles
- PMID: 30177752
- PMCID: PMC6349394
- DOI: 10.1038/s41569-018-0074-0
Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles
Abstract
A large body of evidence indicates that mitochondrial dysfunction has a major role in the pathogenesis of multiple cardiovascular disorders. Over the past 2 decades, extraordinary efforts have been focused on the development of agents that specifically target mitochondria for the treatment of cardiovascular disease. Despite such an intensive wave of investigation, no drugs specifically conceived to modulate mitochondrial functions are currently available for the clinical management of cardiovascular disease. In this Review, we discuss the therapeutic potential of targeting mitochondria in patients with cardiovascular disease, examine the obstacles that have restrained the development of mitochondria-targeting agents thus far, and identify strategies that might empower the full clinical potential of this approach.
Conflict of interest statement
Competing interests
D.A.S. is a consultant to and inventor on patents licensed to CohBar, GlaxoSmithKline, Jumpstart Fertility, Liberty Biosecurity, Life Biosciences, and MetroBiotech. The other authors declare no competing interests.
Figures
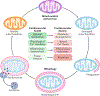
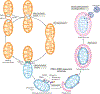
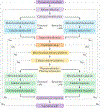

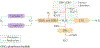
References
-
- Lopez-Crisost C. et al. Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology. Nat. Rev. Cardiol 14, 342–360 (2017). - PubMed
-
- Mehta MM, Weinberg SE & Chandel NS Mitochondrial control of immunity: beyond ATP. Nat. Rev. Immunol 17, 608–620 (2017). - PubMed
-
- Galluzzi L, Kepp O, Trojel-Hansen C & Kroemer G Mitochondrial control of cellular life, stress, and death. Circ. Res 111, 1198–1207 (2012). - PubMed
-
- Galluzzi L et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25, 486–541 (2018). - PMC - PubMed
-
The authors of this paper provide a comprehensive review of the pathways through which mammalian cells die in response to microenvironmental perturbations and propose a unified nomenclature for cell death on the basis of mechanistic and essential (as opposed to morphological and dispensable) aspects of the process.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

