Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer
- PMID: 30177776
- PMCID: PMC6163039
- DOI: 10.1038/s41556-018-0179-z
Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer
Abstract
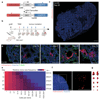
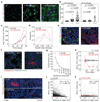
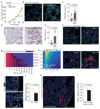
Solid malignancies have been speculated to depend on cancer stem cells (CSCs) for expansion and relapse after therapy. Here we report on quantitative analyses of lineage tracing data from primary colon cancer xenograft tissue to assess CSC functionality in a human solid malignancy. The temporally obtained clone size distribution data support a model in which stem cell function in established cancers is not intrinsically, but is entirely spatiotemporally orchestrated. Functional stem cells that drive tumour expansion predominantly reside at the tumour edge, close to cancer-associated fibroblasts. Hence, stem cell properties change in time depending on the cell location. Furthermore, although chemotherapy enriches for cells with a CSC phenotype, in this context functional stem cell properties are also fully defined by the microenvironment. To conclude, we identified osteopontin as a key cancer-associated fibroblast-produced factor that drives in situ clonogenicity in colon cancer.
Conflict of interest statement
The authors declare no financial interests in the content of this publication.
Figures




Comment in
-
Microenvironmental cues in cancer stemness.Nat Cell Biol. 2018 Oct;20(10):1102-1104. doi: 10.1038/s41556-018-0210-4. Nat Cell Biol. 2018. PMID: 30258126 No abstract available.
References
-
- Vermeulen L, et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science. 2013;342:995–998. - PubMed
-
- Kozar S, et al. Continuous clonal labeling reveals small numbers of functional stem cells in intestinal crypts and adenomas. Cell stem cell. 2013;13:626–633. - PubMed
-
- Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. - PubMed
-
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

