Disentangling astroglial physiology with a realistic cell model in silico
- PMID: 30177844
- PMCID: PMC6120909
- DOI: 10.1038/s41467-018-05896-w
Disentangling astroglial physiology with a realistic cell model in silico
Erratum in
-
Author Correction: Disentangling astroglial physiology with a realistic cell model in silico.Nat Commun. 2019 Nov 4;10(1):5062. doi: 10.1038/s41467-019-12712-6. Nat Commun. 2019. PMID: 31685821 Free PMC article.
Abstract
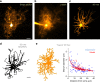
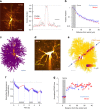
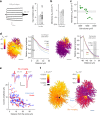
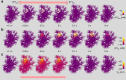
Electrically non-excitable astroglia take up neurotransmitters, buffer extracellular K+ and generate Ca2+ signals that release molecular regulators of neural circuitry. The underlying machinery remains enigmatic, mainly because the sponge-like astrocyte morphology has been difficult to access experimentally or explore theoretically. Here, we systematically incorporate multi-scale, tri-dimensional astroglial architecture into a realistic multi-compartmental cell model, which we constrain by empirical tests and integrate into the NEURON computational biophysical environment. This approach is implemented as a flexible astrocyte-model builder ASTRO. As a proof-of-concept, we explore an in silico astrocyte to evaluate basic cell physiology features inaccessible experimentally. Our simulations suggest that currents generated by glutamate transporters or K+ channels have negligible distant effects on membrane voltage and that individual astrocytes can successfully handle extracellular K+ hotspots. We show how intracellular Ca2+ buffers affect Ca2+ waves and why the classical Ca2+ sparks-and-puffs mechanism is theoretically compatible with common readouts of astroglial Ca2+ imaging.
Conflict of interest statement
The authors declare no competing interests.
Figures

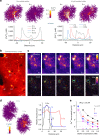
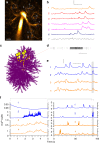
Similar articles
-
Determinants of functional coupling between astrocytes and respiratory neurons in the pre-Bötzinger complex.PLoS One. 2011;6(10):e26309. doi: 10.1371/journal.pone.0026309. Epub 2011 Oct 19. PLoS One. 2011. PMID: 22039458 Free PMC article.
-
GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes.Glia. 2006 Oct;54(5):394-410. doi: 10.1002/glia.20392. Glia. 2006. PMID: 16886203
-
Activation of beta-adrenoceptors opens calcium-activated potassium channels in astroglial cells.Neurochem Int. 2001 Mar;38(3):269-76. doi: 10.1016/s0197-0186(00)00078-4. Neurochem Int. 2001. PMID: 11099786
-
Cross-talk signals in the CNS: role of neurotrophic and hormonal factors, adhesion molecules and intercellular signaling agents in luteinizing hormone-releasing hormone (LHRH)-astroglial interactive network.Front Biosci. 1997 Mar 1;2:d88-125. doi: 10.2741/a177. Front Biosci. 1997. PMID: 9159216 Review.
-
On the electrical passivity of astrocyte potassium conductance.J Neurophysiol. 2021 Oct 1;126(4):1403-1419. doi: 10.1152/jn.00330.2021. Epub 2021 Sep 15. J Neurophysiol. 2021. PMID: 34525325 Free PMC article. Review.
Cited by
-
K+ efflux through postsynaptic NMDA receptors suppresses local astrocytic glutamate uptake.Glia. 2022 May;70(5):961-974. doi: 10.1002/glia.24150. Epub 2022 Jan 27. Glia. 2022. PMID: 35084774 Free PMC article.
-
Monitoring cell membrane recycling dynamics of proteins using whole-cell fluorescence recovery after photobleaching of pH-sensitive genetic tags.Nat Protoc. 2022 Dec;17(12):3056-3079. doi: 10.1038/s41596-022-00732-4. Epub 2022 Sep 5. Nat Protoc. 2022. PMID: 36064755 Review.
-
Computational modeling of the relationship between morphological heterogeneity and functional responses in mouse hippocampal astrocytes.Front Cell Neurosci. 2024 Oct 17;18:1474948. doi: 10.3389/fncel.2024.1474948. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39484184 Free PMC article.
-
Local and CNS-Wide Astrocyte Intracellular Calcium Signaling Attenuation In Vivo with CalExflox Mice.J Neurosci. 2021 May 26;41(21):4556-4574. doi: 10.1523/JNEUROSCI.0085-21.2021. Epub 2021 Apr 26. J Neurosci. 2021. PMID: 33903221 Free PMC article.
-
Astrocytes: new evidence, new models, new roles.Biophys Rev. 2023 Oct 18;15(5):1303-1333. doi: 10.1007/s12551-023-01145-7. eCollection 2023 Oct. Biophys Rev. 2023. PMID: 37975000 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

