Chemical proteomics reveals new targets of cysteine sulfinic acid reductase
- PMID: 30177848
- PMCID: PMC6192846
- DOI: 10.1038/s41589-018-0116-2
Chemical proteomics reveals new targets of cysteine sulfinic acid reductase
Abstract
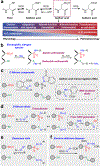
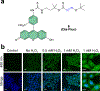
Cysteine sulfinic acid or S-sulfinylation is an oxidative post-translational modification (OxiPTM) that is known to be involved in redox-dependent regulation of protein function but has been historically difficult to analyze biochemically. To facilitate the detection of S-sulfinylated proteins, we demonstrate that a clickable, electrophilic diazene probe (DiaAlk) enables capture and site-centric proteomic analysis of this OxiPTM. Using this workflow, we revealed a striking difference between sulfenic acid modification (S-sulfenylation) and the S-sulfinylation dynamic response to oxidative stress, which is indicative of different roles for these OxiPTMs in redox regulation. We also identified >55 heretofore-unknown protein substrates of the cysteine sulfinic acid reductase sulfiredoxin, extending its function well beyond those of 2-cysteine peroxiredoxins (2-Cys PRDX1-4) and offering new insights into the role of this unique oxidoreductase as a central mediator of reactive oxygen species-associated diseases, particularly cancer. DiaAlk therefore provides a novel tool to profile S-sulfinylated proteins and study their regulatory mechanisms in cells.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures




Comment in
-
Finding S-sulfinylated proteins.Nat Methods. 2018 Nov;15(11):859. doi: 10.1038/s41592-018-0200-2. Nat Methods. 2018. PMID: 30377350 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

