Research Progress in Understanding the Relationship Between Heme Oxygenase-1 and Intracerebral Hemorrhage
- PMID: 30177908
- PMCID: PMC6109777
- DOI: 10.3389/fneur.2018.00682
Research Progress in Understanding the Relationship Between Heme Oxygenase-1 and Intracerebral Hemorrhage
Abstract
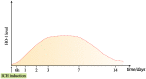
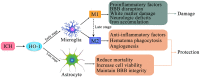
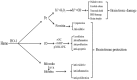
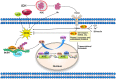
Intracerebral hemorrhage (ICH) is a fatal acute cerebrovascular disease, with a high morbidity and mortality. Following ICH, erythrocytes release heme and several of its metabolites, thereby contributing to brain edema and secondary brain damage. Heme oxygenase is the initial and rate-limiting enzyme of heme catabolism, and the expression of heme oxygenase-1 (HO-1) is rapidly induced following acute brain injury. As HO-1 exerts it effects via various metabolites, its role during ICH remains complex. Therefore, in-depth studies regarding the role of HO-1 in secondary brain damage following ICH may provide a theoretical basis for neuroprotective function after ICH. The present review aims to summarize recent key studies regarding the effects of HO-1 following ICH, as well as its influence on ICH prognosis.
Keywords: heat shock protein 32; heme; heme oxygenase-1; intracerebral hemorrhage; microglia; neurological impairment.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

