Meta-Analysis of Preclinical Studies of Fibrinolytic Therapy for Acute Lung Injury
- PMID: 30177934
- PMCID: PMC6110197
- DOI: 10.3389/fimmu.2018.01898
Meta-Analysis of Preclinical Studies of Fibrinolytic Therapy for Acute Lung Injury
Abstract
Background: Acute lung injury (ALI) is characterized by suppressed fibrinolytic activity in bronchoalveolar lavage fluid (BALF) attributed to elevated plasminogen activator inhibitor-1 (PAI-1). Restoring pulmonary fibrinolysis by delivering tissue-type plasminogen activator (tPA), urokinase plasminogen activator (uPA), and plasmin could be a promising approach.
Objectives: To systematically analyze the overall benefit of fibrinolytic therapy for ALI reported in preclinical studies.
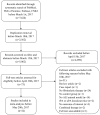
Methods: We searched PubMed, Embase, Web of Science, and CNKI Chinese databases, and analyzed data retrieved from 22 studies for the beneficial effects of fibrinolytics on animal models of ALI.
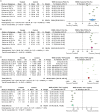
Results: Both large and small animals were used with five routes for delivering tPA, uPA, and plasmin. Fibrinolytics significantly increased the fibrinolytic activity both in the plasma and BALF. Fibrin degradation products in BALF had a net increase of 408.41 ng/ml vs controls (P < 0.00001). In addition, plasma thrombin-antithrombin complexes increased 1.59 ng/ml over controls (P = 0.0001). In sharp contrast, PAI-1 level in BALF decreased 21.44 ng/ml compared with controls (P < 0.00001). Arterial oxygen tension was improved by a net increase of 15.16 mmHg, while carbon dioxide pressure was significantly reduced (11.66 mmHg, P = 0.0001 vs controls). Additionally, fibrinolytics improved lung function and alleviated inflammation response: the lung wet/dry ratio was decreased 1.49 (P < 0.0001 vs controls), lung injury score was reduced 1.83 (P < 0.00001 vs controls), and BALF neutrophils were lesser (3 × 104/ml, P < 0.00001 vs controls). The mortality decreased significantly within defined study periods (6 h to 30 days for mortality), as the risk ratio of death was 0.2-fold of controls (P = 0.0008).
Conclusion: We conclude that fibrinolytic therapy may be effective pharmaceutic strategy for ALI in animal models.
Keywords: fibrinolytic agents; interventions; lung diseases; molecular therapy; preclinical study.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

