Nanomedicine: Principles, Properties, and Regulatory Issues
- PMID: 30177965
- PMCID: PMC6109690
- DOI: 10.3389/fchem.2018.00360
Nanomedicine: Principles, Properties, and Regulatory Issues
Abstract
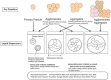
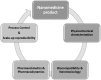
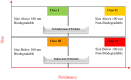
Several scientific areas have benefited significantly from the introduction of nanotechnology and the respective evolution. This is especially noteworthy in the development of new drug substances and products. This review focuses on the introduction of nanomedicines in the pharmaceutical market, and all the controversy associated to basic concepts related to these nanosystems, and the numerous methodologies applied for enhanced knowledge. Due to the properties conferred by the nanoscale, the challenges for nanotechnology implementation, specifically in the pharmaceutical development of new drug products and respective regulatory issues are critically discussed, mainly focused on the European Union context. Finally, issues pertaining to the current applications and future developments are presented.
Keywords: nanomaterials; nanomedicine; nanotechnology; nanotoxicology; pharmaceutical development.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

