Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial
- PMID: 30178032
- PMCID: PMC6440720
- DOI: 10.1001/jamaoncol.2018.3277
Nab-Paclitaxel and Gemcitabine as First-line Treatment of Advanced or Metastatic Cholangiocarcinoma: A Phase 2 Clinical Trial
Abstract
Importance: Gemcitabine with platinum has limited efficacy for treatment of advanced cholangiocarcinoma, necessitating an evaluation of alternative drug combinations. Recent evidence suggests that paclitaxel may potentiate gemcitabine activity.
Objective: To evaluate whether gemcitabine plus nanoparticle albumin-bound (nab)-paclitaxel is safe and effective for treatment of advanced cholangiocarcinoma.
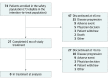
Design, setting, and participants: This single-arm, 2-stage, phase 2 clinical trial was conducted at 23 community and academic centers across the United States and Europe. Patients aged 18 years or older enrolled between September 2014 and March 2016 had confirmed advanced or metastatic cholangiocarcinoma without prior systemic therapy, and had an Eastern Cooperative Oncology Group Performance Status score of 0 to 1 and a Child-Pugh score less than 8. Previous surgery, radiation, or liver-directed therapies were permitted.
Interventions: Patients received intravenous nab-paclitaxel, 125 mg/m2, followed by gemcitabine, 1000 mg/m2, on days 1, 8, and 15 of each 28-day treatment cycle until disease progression or unacceptable toxic effects.
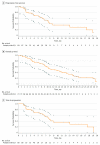
Main outcomes and measures: The primary outcome was improvement in 6-month progression-free survival (PFS) rate (null and alternative hypotheses of 55% and 70%, respectively) in the evaluable population. Secondary outcomes included median overall survival (OS), PFS, time to progression, best overall response rate, disease control rate, safety and toxicity, and association of change in carbohydrate antigen 19-9 with survival.
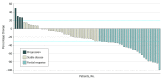
Results: Seventy-four patients with a median age of 62 (range, 36-87) years, including 44 women (60%), were enrolled. Patients received a median of 6 (range, 1-18) treatment cycles, and the median follow-up was 10.2 (range, 0.6-27.3) months. The observed 6-month PFS rate of 61% (95% CI, 48%-73%) did not favor the alternative hypothesis. Median PFS was 7.7 (95% CI, 5.4-13.1) months, median OS was 12.4 (95% CI, 9.2-15.9) months, and median time to progression was 7.7 (95% CI, 6.1-13.1) months. The confirmed best overall response rate and disease control rate were 30% and 66%, respectively. Hazard ratios for an association between a change in serum carbohydrate antigen 19-9 and median PFS as well as median OS were 2.02 (95% CI, 0.86-4.75) (P = .10) and 1.54 (95% CI, 0.64-3.71) (P = .34), respectively. The most common treatment-related hematologic and nonhematologic adverse events at grade 3 or higher were neutropenia (43%) and fatigue (14%), respectively.
Conclusions and relevance: Although the trial did not meet its primary efficacy end point, the results indicate that a nab-paclitaxel plus gemcitabine regimen was well tolerated and may be an alternative option to current therapeutic approaches for advanced cholangiocarcinoma.
Trial registration: ClinicalTrials.gov identifier: NCT02181634.
Conflict of interest statement
Figures
References
-
- Malka D, Cervera P, Foulon S, et al. ; BINGO investigators . Gemcitabine and Oxaliplatin With or Without Cetuximab in Advanced Biliary-Tract Cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15(8):819-828. doi: 10.1016/S1470-2045(14)70212-8 - DOI - PMC - PubMed
-
- Frese KK, Neesse A, Cook N, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2(3):260-269. doi: 10.1158/2159-8290.CD-11-0242 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

