Neurotoxicity after CTL019 in a pediatric and young adult cohort
- PMID: 30178481
- PMCID: PMC6444896
- DOI: 10.1002/ana.25315
Neurotoxicity after CTL019 in a pediatric and young adult cohort
Abstract
Objective: To characterize the incidence and clinical characteristics of neurotoxicity in the month following CTL019 infusion in children and young adults, to define the relationship between neurotoxicity and cytokine release syndrome (CRS), and to identify predictive biomarkers for development of neurotoxicity following CTL019 infusion.
Methods: We analyzed data on 51 subjects, 4 to 22 years old, who received CTL019, a chimeric antigen receptor-modified T-cell therapy against CD19, between January 1, 2010 and December 1, 2015 through a safety/feasibility clinical trial (NCT01626495) at our institution. We recorded incidence of significant neurotoxicity (encephalopathy, seizures, and focal deficits) and CRS, and compared serum cytokine levels in the first month postinfusion between subjects who did and did not develop neurotoxicity.
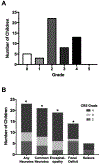
Results: Neurotoxicity occurred in 23 of 51 subjects (45%, 95% confidence interval = 31-60%) and was positively associated with higher CRS grade (p < 0.0001) but was not associated with demographic characteristics or prior oncologic treatment history. Serum interleukin (IL)-2, IL-15, soluble IL-4, and hepatocyte growth factor concentrations were higher in subjects with neurotoxicity than those with isolated CRS. Differences in peak levels of select cytokines including IL-12 and soluble tumor necrosis factor receptor-1 within the first 3 days were seen in subjects with neurotoxicity.
Interpretation: Neurotoxicity is common after CTL019 infusion in children and young adults, and is associated with higher CRS grade. Differences in serum cytokine profiles between subjects with neurotoxicity and those with isolated CRS suggest unique pathophysiological mechanisms. Serum cytokine profiles in the first 3 days postinfusion may help identify children and young adults at risk for neurotoxicity, and may provide a foundation for investigation into potential mitigation strategies. Ann Neurol 2018;84:537-546.
© 2018 American Neurological Association.
Conflict of interest statement
Figures


References
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial [Internet]. Lancet 2015;385(9967):517–528.Available from: http://www.sciencedirect.com/science/article/pii/S0140673614614033%5Cnht... - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

