Metformin reverses early cortical network dysfunction and behavior changes in Huntington's disease
- PMID: 30179155
- PMCID: PMC6156080
- DOI: 10.7554/eLife.38744
Metformin reverses early cortical network dysfunction and behavior changes in Huntington's disease
Abstract
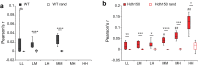
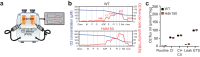
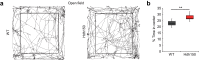
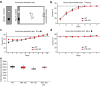
Catching primal functional changes in early, 'very far from disease onset' (VFDO) stages of Huntington's disease is likely to be the key to a successful therapy. Focusing on VFDO stages, we assessed neuronal microcircuits in premanifest Hdh150 knock-in mice. Employing in vivo two-photon Ca2+ imaging, we revealed an early pattern of circuit dysregulation in the visual cortex - one of the first regions affected in premanifest Huntington's disease - characterized by an increase in activity, an enhanced synchronicity and hyperactive neurons. These findings are accompanied by aberrations in animal behavior. We furthermore show that the antidiabetic drug metformin diminishes aberrant Huntingtin protein load and fully restores both early network activity patterns and behavioral aberrations. This network-centered approach reveals a critical window of vulnerability far before clinical manifestation and establishes metformin as a promising candidate for a chronic therapy starting early in premanifest Huntington's disease pathogenesis long before the onset of clinical symptoms.
Keywords: C. elegans; Huntington disease; cortical microcircuits; in vivo calcium imaging; metformin; mouse; neuronal hyperactivity; neuroscience.
© 2018, Arnoux et al.
Conflict of interest statement
IA, MW, NG, JK, HW, NO, SW, PN, CC, OM, SB, KM, DB, KR, RL, JL, EW, AM, SK, SS, AS No competing interests declared
Figures
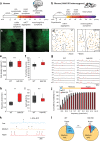
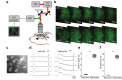
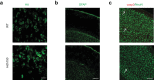

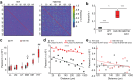
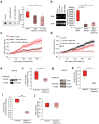
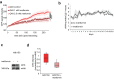


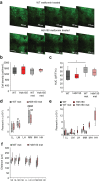
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

