Tale of two vaccines: differences in response to herpes zoster vaccines
- PMID: 30179221
- PMCID: PMC6159952
- DOI: 10.1172/JCI123217
Tale of two vaccines: differences in response to herpes zoster vaccines
Abstract
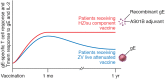
About one-third of the US population will develop herpes zoster (HZ, commonly known as shingles) over a lifetime, while two-thirds will not. It is not clear exactly why certain people are susceptible to HZ; however, we may be coming closer to an answer. In this issue of the JCI, a study by Levin et al. provides important details concerning pathogenesis of and protection from HZ. The authors characterized differences in the immunologic responses induced by two HZ vaccines, the live attenuated zoster vaccine (ZV) and the more recently developed adjuvanted varicella-zoster virus (VZV) glycoprotein E (gE) subunit herpes zoster vaccine (HZ/su), in vaccine-naive subjects and those previously vaccinated with HZ. The observed differences in responses paralleled the observed clinical protection of the two zoster vaccines, with HZ/su being superior to HZ. Together, these results seem to explain immunologically why the new subunit vaccine outperforms the live vaccine. These differences may also provide clues as to why HZ develops in the first place.
Conflict of interest statement
Figures
Comment on
-
Th1 memory differentiates recombinant from live herpes zoster vaccines.J Clin Invest. 2018 Oct 1;128(10):4429-4440. doi: 10.1172/JCI121484. Epub 2018 Jul 19. J Clin Invest. 2018. PMID: 30024861 Free PMC article. Clinical Trial.
References
-
- Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2(7892):1288–1290. - PubMed
-
- Gershon AA, et al. Live attenuated varicella vaccine. Efficacy for children with leukemia in remission. JAMA. 1984;252(3):355–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

