Extracellular histones are the ligands for the uptake of exosomes and hydroxyapatite-nanoparticles by tumor cells via syndecan-4
- PMID: 30179249
- PMCID: PMC6188801
- DOI: 10.1002/1873-3468.13236
Extracellular histones are the ligands for the uptake of exosomes and hydroxyapatite-nanoparticles by tumor cells via syndecan-4
Abstract
The mechanisms by which exosomes (nano-vesicular messengers of cells) are taken up by recipient cells are poorly understood. We hypothesized that histones associated with these nanoparticles are the ligands which facilitate their interaction with cell surface syndecan-4 (SDC4) to mediate their uptake. We show that the incubation with fetuin-A (exosome-associated proteins) and histones mediates the uptake of exosomes that are normally not endocytosed. Similarly, hydroxyapatite-nanoparticles incubated with fetuin-A and histones (FNH) are internalized by tumor cells, while nanoparticles incubated with fetuin-A alone (FN) are not. The uptake of exosomes and FNH, both of which move to the perinuclear region of the cell, is attenuated in SDC4-knockdown cells. Data show that FNH can compete with exosomes for uptake and that both use SDC4 as uptake receptors.
Keywords: exosomes; histones; hydroxyapatite; nanoparticles and syndecan-4.
© 2018 Federation of European Biochemical Societies.
Figures

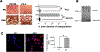

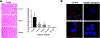



Similar articles
-
Fetuin-A associates with histones intracellularly and shuttles them to exosomes to promote focal adhesion assembly resulting in rapid adhesion and spreading in breast carcinoma cells.Exp Cell Res. 2014 Nov 1;328(2):388-400. doi: 10.1016/j.yexcr.2014.08.037. Epub 2014 Sep 4. Exp Cell Res. 2014. PMID: 25194507 Free PMC article.
-
Phosphorylation-regulated phase separation of syndecan-4 and syntenin promotes the biogenesis of exosomes.Cell Prolif. 2024 Oct;57(10):e13645. doi: 10.1111/cpr.13645. Epub 2024 Apr 11. Cell Prolif. 2024. PMID: 38601993 Free PMC article.
-
Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity.Proc Natl Acad Sci U S A. 2013 Oct 22;110(43):17380-5. doi: 10.1073/pnas.1304266110. Epub 2013 Oct 7. Proc Natl Acad Sci U S A. 2013. PMID: 24101524 Free PMC article.
-
Correlation of syndecan gene amplification with metastatic potential and clinical outcomes in carcinomas.Am J Physiol Cell Physiol. 2024 Aug 1;327(2):C380-C386. doi: 10.1152/ajpcell.00270.2024. Epub 2024 Jul 2. Am J Physiol Cell Physiol. 2024. PMID: 38953842 Review.
-
Internalization of Exosomes through Receptor-Mediated Endocytosis.Mol Cancer Res. 2019 Feb;17(2):337-347. doi: 10.1158/1541-7786.MCR-18-0891. Epub 2018 Nov 28. Mol Cancer Res. 2019. PMID: 30487244 Review.
Cited by
-
Extracellular vesicles (EVs)' journey in recipient cells: from recognition to cargo release.J Zhejiang Univ Sci B. 2024 Aug 15;25(8):633-655. doi: 10.1631/jzus.B2300566. J Zhejiang Univ Sci B. 2024. PMID: 39155778 Free PMC article. Review.
-
Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate.Cells. 2022 Apr 19;11(9):1375. doi: 10.3390/cells11091375. Cells. 2022. PMID: 35563681 Free PMC article. Review.
-
The emerging roles and mechanisms of exosomal non-coding RNAs in the mutual regulation between adipose tissue and other related tissues in obesity and metabolic diseases.Front Endocrinol (Lausanne). 2022 Aug 18;13:975334. doi: 10.3389/fendo.2022.975334. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060952 Free PMC article. Review.
-
Syndecan-4 is a maestro of gastric cancer cell invasion and communication that underscores poor survival.Proc Natl Acad Sci U S A. 2023 May 16;120(20):e2214853120. doi: 10.1073/pnas.2214853120. Epub 2023 May 8. Proc Natl Acad Sci U S A. 2023. PMID: 37155874 Free PMC article.
-
Exosome-Mediated Antigen Delivery: Unveiling Novel Strategies in Viral Infection Control and Vaccine Design.Vaccines (Basel). 2024 Mar 7;12(3):280. doi: 10.3390/vaccines12030280. Vaccines (Basel). 2024. PMID: 38543914 Free PMC article. Review.
References
-
- Syn N, Wang L, Sethi G, Thiery JP & Goh BC (2016) Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance, Trends in pharmacological sciences. 37, 606–617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

