Pharmacological Inhibition of CCR2/5 Signaling Prevents and Reverses Alcohol-Induced Liver Damage, Steatosis, and Inflammation in Mice
- PMID: 30179264
- PMCID: PMC6393202
- DOI: 10.1002/hep.30249
Pharmacological Inhibition of CCR2/5 Signaling Prevents and Reverses Alcohol-Induced Liver Damage, Steatosis, and Inflammation in Mice
Abstract
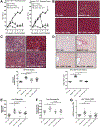
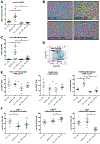
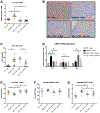
Kupffer cell and macrophage (MØ) activation contributes to steatosis, inflammation, and fibrosis in alcoholic liver disease (ALD). We found increased frequency of MØ, T cells, and expression of C-C chemokine receptor type 2 (Ccr2) and C-C chemokine receptor type 5 (Ccr5) in the livers of patients with ALD, and increased circulating chemokines, C-C chemokine ligand types 2 (CCL2), and C-C chemokine ligand types 5 (CCL5) in patients with alcoholic hepatitis. We hypothesized that inhibition of CCL2 signaling with the dual CCR2/5 inhibitor, cenicriviroc (CVC), would attenuate ALD. In a mouse model of ALD, liver injury (alanine aminotransferase [ALT]) and steatosis were prevented by CVC whether administered as "prevention" throughout the alcohol feeding or as "treatment" started after the development of ALD. Alcohol-induced increases in early liver fibrosis markers (sirius red, hydroxyproline, and collagen-1) were normalized by both modes of CVC administration. We found that prevention and treatment with CVC reversed alcohol-related increases in liver mRNA and protein expression of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and CCL2. CVC administration regimens prevented the increase in infiltrating MØ (F4/80lo CD11bhi ) and reduced proinflammatory Ly6Chi MØ in livers of alcohol-fed mice. CVC increased liver T-cell numbers and attenuated Il-2 expression without an effect on CD69+ or CD25+ T-cell expression. In vitro, CVC inhibited CCL2-induced increases in hepatocyte fatty acid synthase (Fasn) and adipose differentiation-related protein (Adrp), whereas it augmented acyl-coenzyme A oxidase 1 (Acox-1), proliferator-activated receptor gamma co-activator alpha (Pgc1α) and uncoupling protein 2 expression, suggesting mechanisms for attenuated hepatocyte steatosis. We found that CCL2 and CCL5 sensitized hepatocytes to lipopolysaccharide-induced liver injury (TNF-α, ALT, and lactate dehydrogenase release). Alcohol feeding induced apoptosis (poly ADP-ribose polymerase [PARP] and caspase-3 [CASP-3] cleavage) and pyroptosis (gasdermin D [GSDMD] cleavage) in livers, and CVC prevented both of these forms of cell death. Conclusion: Together, our data demonstrate preclinical evidence for CCR2/CCR5 inhibition with CVC as a potent intervention to ameliorate alcohol-induced steatohepatitis and liver damage.
© 2018 by the American Association for the Study of Liver Diseases.
Figures



References
-
- O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology 2010;51:307–328. - PubMed
-
- Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis 2012;16:659–666. - PubMed
-
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360:2758–2769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

