A method to reduce variability in scoring antibody-mediated rejection in renal allografts: implications for clinical trials - a retrospective study
- PMID: 30179275
- PMCID: PMC6328317
- DOI: 10.1111/tri.13340
A method to reduce variability in scoring antibody-mediated rejection in renal allografts: implications for clinical trials - a retrospective study
Abstract
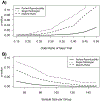
Poor reproducibility in scoring antibody-mediated rejection (ABMR) using the Banff criteria might limit the use of histology in clinical trials. We evaluated the reproducibility of Banff scoring of 67 biopsies by six renal pathologists at three institutions. Agreement by any two pathologists was poor: 44.8-65.7% for glomerulitis, 44.8-67.2% for peritubular capillaritis, and 53.7-80.6% for chronic glomerulopathy (cg). All pathologists agreed on cg0 (n = 20) and cg3 (n = 9) cases, however, many disagreed on scores of cg1 or cg2. The range for the incidence of composite diagnoses by individual pathologists was: 16.4-22.4% for no ABMR; 17.9-47.8% for active ABMR; and 35.8-59.7% for chronic, active antibody-mediated rejection (cABMR). A "majority rules" approach was then tested in which the scores of three pathologists were used to reach an agreement. This increased consensus both for individual scores (ex. 67.2-77.6% for cg) and for composite diagnoses (ex. 74.6-86.6% cABMR). Modeling using these results showed that differences in individual scoring could affect the outcome assessment in a mock study of cABMR. We conclude that the Banff schema has high variability and a majority rules approach could be used to adjudicate differences between pathologists and reduce variability in scoring in clinical trials.
Keywords: kidney clinical; rejection.
© 2018 Steunstichting ESOT.
Conflict of interest statement
Figures

References
-
- Gough J, Rush D, Jeffery J, et al. Reproducibility of the Banff schema in reporting protocol biopsies of stable renal allografts. Nephrol Dial Transplant. 2002;17(6):1081–1084. - PubMed
-
- Veronese FV, Manfro RC, Roman FR, et al. Reproducibility of the Banff classification in subclinical kidney transplant rejection. Clin Transplant. 2005;19(4):518–521. - PubMed
-
- Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. - PMC - PubMed
-
- Cohen J A Coefficient of Agreement for Nominal Scales. Educational and Psychological Measurement. 1960;20(1):37–46.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

