Tumor hypoxia directed multimodal nanotherapy for overcoming drug resistance in renal cell carcinoma and reprogramming macrophages
- PMID: 30179778
- PMCID: PMC6414719
- DOI: 10.1016/j.biomaterials.2018.08.053
Tumor hypoxia directed multimodal nanotherapy for overcoming drug resistance in renal cell carcinoma and reprogramming macrophages
Abstract
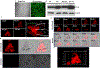
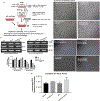
Drug resistance is one of the significant clinical burden in renal cell carcinoma (RCC). The development of drug resistance is attributed to many factors, including impairment of apoptosis, elevation of carbonic anhydrase IX (CA IX, a marker of tumor hypoxia), and infiltration of tumorigenic immune cells. To alleviate the drug resistance, we have used Sorafenib (Sor) in combination with tumor hypoxia directed nanoparticle (NP) loaded with a new class of apoptosis inducer, CFM 4.16 (C4.16), namely CA IX-C4.16. The NP is designed to selectively deliver the payload to the hypoxic tumor (core), provoke superior cell death in parental (WT) and Everolimus-resistant (Evr-res) RCC and selectively downmodulate tumorigenic M2-macrophage. Copper-free 'click' chemistry was utilized for conjugating SMA-TPGS with Acetazolamide (ATZ, a CA IX-specific targeting ligand). The NP was further tagged with a clinically approved NIR dye (S0456) for evaluating hypoxic tumor core penetration and organ distribution. Imaging of tumor spheroid treated with NIR dye-labeled CA IX-SMA-TPGS revealed remarkable tumor core penetration that was modulated by CA IX-mediated targeting in hypoxic-A498 RCC cells. The significant cell killing effect with synergistic combination index (CI) of CA IX-C4.16 and Sor treatment suggests efficient reversal of Evr-resistance in A498 cells. The CA IX directed nanoplatform in combination with Sor has shown multiple benefits in overcoming drug resistance through (i) inhibition of p-AKT, (ii) upregulation of tumoricidal M1 macrophages resulting in induction of caspase 3/7 mediated apoptosis of Evr-res A498 cells in macrophage-RCC co-culturing condition, (iii) significant in vitro and in vivo Evr-res A498 tumor growth inhibition as compared to individual therapy, and (iv) untraceable liver and kidney toxicity in mice. Near-infrared (NIR) imaging of CA IX-SMA-TPGS-S0456 in Evr-res A498 RCC model exhibited significant accumulation of CA IX-oligomer in tumor core with >3-fold higher tumor uptake as compared to control. In conclusion, this proof-of-concept study demonstrates versatile tumor hypoxia directed nanoplatform that can work in synergy with existing drugs for reversing drug-resistance in RCC accompanied with re-education of tumor-associated macrophages, that could be applied universally for several hypoxic tumors.
Keywords: Carbonic anhydrase IX; Everolimus; Macrophage modulation; Nano-therapy; Overcoming drug resistance; Renal cell carcinoma; Tumor core penetration; Tumor hypoxia targeting.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest
There are no conflicts to declare.
Figures


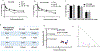



References
-
- Creighton CJ, Morgan M, Gunaratne PH, Wheeler DA, Gibbs RA, Gordon Robertson A, Chu A, Beroukhim R, Cibulskis K, Signoretti S, Vandin Hsin-Ta Wu F, Raphael BJ, Verhaak RGW, Tamboli P, Torres-Garcia W, Akbani R, Weinstein JN, Reuter V, Hsieh JJ, Rose Brannon A, Ari Hakimi A, Jacobsen A, Ciriello G, Reva B, Ricketts CJ, Marston Linehan W, Stuart JM, Kimryn Rathmell W, Shen H, Laird PW, Muzny D, Davis C, Xi L, Chang K, Kakkar N, Treviño LR, Benton S, Reid JG, Morton D, Doddapaneni H, Han Y, Lewis L, Dinh H, Kovar C, Zhu Y, Santibanez J, Wang M, Hale W, Kalra D, Getz G, Lawrence MS, Sougnez C, Carter SL, Sivachenko A, Lichtenstein L, Stewart C, Voet D Fisher Sheila, Gabriel Stacey B., Lander E, Schumacher Steve E., Tabak B, Saksena Gordon, Onofrio RC, Cherniack AD, Gentry Jeff, Ardlie K, Sougnez Carrie, Gabriel SB, Meyerson M, Chun H-JE, Mungall AJ, Sipahimalani P, Stoll D, Ally A, Balasundaram M, Butterfield YSN, Carlsen R, Carter C, Chuah E, Coope RJN, Dhalla N, Gorski S, Guin R, Hirst C, Hirst M, Holt RA, Lebovitz C, Lee D, Li HI, Mayo M, Moore RA, Pleasance E, Plettner P, Schein JE, Shafiei A, Slobodan JR, Tam A, Thiessen N, Varhol RJ, Wye N, Zhao Y, Birol I, Jones SJM, Marra MA, Auman JT, Tan D, Jones CD, Hoadley KA, Mieczkowski PA, Mose LE, Jefferys SR, Topal MD, Liquori C, Turman YJ, Shi Y, Waring S, Buda E, Walsh J, Wu J, Bodenheimer T, Hoyle AP, Simons JV, Soloway MG, Balu S, Parker JS, Neil Hayes D, Perou CM, Kucherlapati R, Park P, Triche T Jr, Weisenberger DJ, Lai PH, Bootwalla MS, Maglinte DT, Mahurkar S, Berman BP, Van Den Berg DJ, Cope L, Baylin SB, Noble MS, DiCara D, Zhang H, Cho J, Heiman DI, Gehlenborg N, Mallard W, Lin P, Frazer S, Stojanov P, Liu Y, Zhou L, Kim J, Chin L, Vandin F, Wu H-T, Benz C, Yau C, Reynolds SM, Shmulevich I, Verhaak RGW, Vegesna R, Kim H, Zhang W, Cogdell D, Jonasch E, Ding Z, Lu Y, Zhang N, Unruh AK, Casasent TD, Wakefield C, Tsavachidou D, Mills GB, Schultz N, Antipin Y, Gao J, Cerami E, Gross B, Arman Aksoy B, Sinha R, Weinhold N, Onur Sumer S, Taylor BS, Shen R, Ostrovnaya I, Berger MF, Ladanyi M, Sander C, Fei SS, Stout A, Spellman PT, Rubin DL, Liu TT, Ng S, Paull EO, Carlin D, Goldstein T, Waltman P, Ellrott K, Zhu J, Haussler D, Xiao W, Shelton C, Gardner J, Penny R, Sherman M, Mallery D, Morris S, Paulauskis J, Burnett K, Shelton T, Kaelin WG, Choueiri T, Atkins MB, Curley E, Tickoo S, Thorne L, Boice L, Huang M, Fisher JC, Vocke CD, Peterson J, Worrell R, Merino MJ, Schmidt LS, Czerniak BA, Aldape KD, Wood CG, Boyd J, Weaver J, Iacocca MV, Petrelli N, Witkin G, Brown J, Czerwinski C, Huelsenbeck-Dill L, Rabeno B, Myers J, Morrison C, Bergsten J, Eckman J, Harr J, Smith C, Tucker K, Anne Zach L, Bshara W, Gaudioso C, Dhir R, Maranchie J, Nelson J, Parwani A, Potapova O, Fedosenko K, Cheville JC, Houston Thompson R, Mosquera JM, Rubin MA, Blute ML, Pihl T, Jensen M, Sfeir R, Kahn A, Chu A, Kothiyal P, Snyder E, Pontius J, Ayala B, Backus M, Walton J, Baboud J, Berton D, Nicholls M, Srinivasan D, Raman R, Girshik S, Kigonya P, Alonso S, Sanbhadti R, Barletta S, Pot D, Sheth M, Demchok JA, Davidsen T, Wang Z, Yang L, Tarnuzzer RW, Zhang J, Eley G, Ferguson ML, Mills Shaw KR, Guyer MS, Ozenberger BA, Sofia HJ., Comprehensive molecular characterization of clear cell renal cell carcinoma, Nature. 499 (2013) 43–49. doi:10.1038/nature12222. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

