NLR Family Pyrin Domain-Containing 3 Inflammasome Activation in Hepatic Stellate Cells Induces Liver Fibrosis in Mice
- PMID: 30180270
- PMCID: PMC6351190
- DOI: 10.1002/hep.30252
NLR Family Pyrin Domain-Containing 3 Inflammasome Activation in Hepatic Stellate Cells Induces Liver Fibrosis in Mice
Abstract
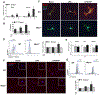
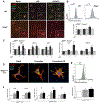
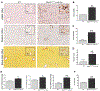
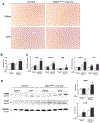
The NLR family pyrin domain-containing 3 (NLRP3) inflammasome plays an important role in liver fibrosis (LF) development. However, the mechanisms involved in NLRP3-induced fibrosis are unclear. Our aim was to test the hypothesis that the NLRP3 inflammasome in hepatic stellate cells (HSCs) can directly regulate their activation and contribute to LF. Primary HSCs isolated from wild-type (WT), Nlrp3-/- , or Nlrp3L351PneoR knock-in crossed to inducible (estrogen receptor Cre-CreT) mice were incubated with lipopolysaccharide (LPS) and adenosine triphosphate (ATP), or 4OH-tamoxifen, respectively. HSC-specific Nlrp3L351P knock-in mice were generated by crossing transgenic mice expressing lecithin retinol acyltransferase (Lrat)-driven Cre and maintained on standard rodent chow for 6 months. Mice were then sacrificed; liver tissue and serum were harvested. Nlrp3 inflammasome activation along with HSC phenotype and fibrosis were assessed by RT-PCR, western blotting, fluorescence-activated cell sorting (FACS), enzyme-linked immunosorbent assay, immunofluorescence (IF), and immunohistochemistry (IHC). Stimulated WT HSCs displayed increased levels of NLRP3 inflammasome-induced reactive oxygen species (ROS) production and cathepsin B activity, accompanied by an up-regulation of mRNA and protein levels of fibrotic makers, an effect abrogated in Nlrp3-/- HSCs. Nlrp3L351P CreT HSCs also showed elevated mRNA and protein expression of fibrotic markers 24 hours after inflammasome activation induced with 4-hydroxytamoxifen (4OHT). Protein and mRNA expression levels of fibrotic markers were also found to be increased in isolated HSCs and whole liver tissue from Nlrp3L351P Lrat Cre mice compared to WT. Liver sections from 24-week-old NlrpL351P Lrat Cre mice showed fibrotic changes with increased alpha smooth muscle actin (αSMA) and desmin-positive cells and collagen deposition, independent of inflammatory infiltrates; these changes were also observed after LPS challenge in 8-week-old NlrpL351P Lrat Cre mice. Conclusion: Our results highlight a direct role for the NLRP3 inflammasome in the activation of HSCs directly triggering LF.
© 2018 by the American Association for the Study of Liver Diseases.
Figures


References
-
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012;481:278–286. - PubMed
-
- Gross CJ, Mishra R, Schneider KS, Medard G, Wettmarshausen J, Dittlein DC, Shi H, et al. K(+) Efflux-Independent NLRP3 Inflammasome Activation by Small Molecules Targeting Mitochondria. Immunity 2016;45:761–773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

