A two-hybrid antibody micropattern assay reveals specific in cis interactions of MHC I heavy chains at the cell surface
- PMID: 30180933
- PMCID: PMC6125123
- DOI: 10.7554/eLife.34150
A two-hybrid antibody micropattern assay reveals specific in cis interactions of MHC I heavy chains at the cell surface
Abstract
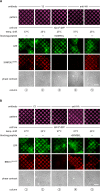
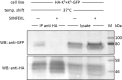
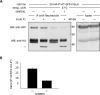
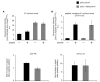
We demonstrate a two-hybrid assay based on antibody micropatterns to study protein-protein interactions at the cell surface of major histocompatibility complex class I (MHC I) proteins. Anti-tag and conformation-specific antibodies are used for individual capture of specific forms of MHC I proteins that allow for location- and conformation-specific analysis by fluorescence microscopy. The assay is used to study the in cis interactions of MHC I proteins at the cell surface under controlled conditions and to define the involved protein conformations. Our results show that homotypic in cis interactions occur exclusively between MHC I free heavy chains, and we identify the dissociation of the light chain from the MHC I protein complex as a condition for MHC I in cis interactions. The functional role of these MHC I protein-protein interactions at the cell surface needs further investigation. We propose future technical developments of our two-hybrid assay for further analysis of MHC I protein-protein interactions.
Keywords: cell biology; endocytosis; immunology; in cis interactions; inflammation; major histocompatibility complex molecules; membrane proteins; protein micropatterns.
© 2018, Dirscherl et al.
Conflict of interest statement
CD, ZH, VR, CJ, SS No competing interests declared
Figures




References
-
- Allen RL, O'Callaghan CA, McMichael AJ, Bowness P. Cutting edge: HLA-B27 can form a novel beta 2-microglobulin-free heavy chain homodimer structure. Journal of Immunology. 1999;162:5045–5048. - PubMed
-
- Bodnár A, Bacsó Z, Jenei A, Jovin TM, Edidin M, Damjanovich S, Matkó J. Class I HLA oligomerization at the surface of B cells is controlled by exogenous beta(2)-microglobulin: implications in activation of cytotoxic T lymphocytes. International Immunology. 2003;15:331–339. doi: 10.1093/intimm/dxg042. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

