International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease
- PMID: 30181172
- PMCID: PMC6238190
- DOI: 10.1182/blood-2018-07-862334
International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease
Abstract
Castleman disease (CD) describes a group of heterogeneous hematologic disorders with characteristic histopathological features. CD can present with unicentric or multicentric (MCD) regions of lymph node enlargement. Some cases of MCD are caused by human herpesvirus-8 (HHV-8), whereas others are HHV-8-negative/idiopathic (iMCD). Treatment of iMCD is challenging, and outcomes can be poor because no uniform treatment guidelines exist, few systematic studies have been conducted, and no agreed upon response criteria have been described. The purpose of this paper is to establish consensus, evidence-based treatment guidelines based on the severity of iMCD to improve outcomes. An international Working Group of 42 experts from 10 countries was convened by the Castleman Disease Collaborative Network to establish consensus guidelines for the management of iMCD based on published literature, review of treatment effectiveness for 344 cases, and expert opinion. The anti-interleukin-6 monoclonal antibody siltuximab (or tocilizumab, if siltuximab is not available) with or without corticosteroids is the preferred first-line therapy for iMCD. In the most severe cases, adjuvant combination chemotherapy is recommended. Additional agents are recommended, tailored by disease severity, as second- and third-line therapies for treatment failures. Response criteria were formulated to facilitate the evaluation of treatment failure or success. These guidelines should help treating physicians to stratify patients based on disease severity in order to select the best available therapeutic option. An international registry for patients with CD (ACCELERATE, #NCT02817997) was established in October 2016 to collect patient outcomes to increase the evidence base for selection of therapies in the future.
Conflict of interest statement
Conflict-of-interest disclosure: F.v.R. has received research funding from Bristol Myers-Squibb and Janssen Pharmaceuticals and has served on advisory boards from Janssen Pharmaceuticals. D.C.F. has received research funding from Janssen Pharmaceuticals. R.W. and C.C. have received research funding from Janssen Pharmaceuticals and served on advisory boards for Janssen Pharmaceuticals. P.V. has served on advisory boards for Janssen Pharmaceuticals. S.F. has received consultancy fees and speaker honoraria and served on advisory boards for Janssen Pharmaceuticals. A.F. has received honoraria from Janssen Pharmaceuticals. T.S.U. has received research funding Genentech, Merck, and Celgene via Cooperative Research and Development Agreements with the National Cancer Institute and has a patent for an immunomodulatory compound for KSHV malignancies (Inst). R.K. has received research funding from Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, and Konica Minolta, consultant fees from LOXO, Actuate Therapeutics, Genentech, and NeoMed, as well as speaker fees from Roche, and has an ownership interest in Curematch, Inc. D. Simpson has received honoraria and research funding from Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures
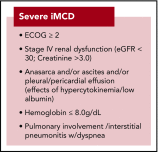

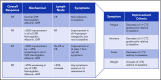
Comment in
-
Do you know TAFRO?Blood. 2018 Nov 15;132(20):2109-2110. doi: 10.1182/blood-2018-09-875112. Blood. 2018. PMID: 30442745 No abstract available.
References
-
- Castleman B, Towne VW. Case records of the Massachusetts General Hospital; weekly clinicopathological exercises; founded by Richard C. Cabot. N Engl J Med. 1954;251(10):396-400. - PubMed
-
- Flendrig J. Benign giant lymphoma: the clincal signs and symptoms and the morphological aspects. Folia Med (Plovdiv). 1969;12:119-120.
-
- Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29(3):670-683. - PubMed
-
- Gaba AR, Stein RS, Sweet DL, Variakojis D. Multicentric giant lymph node hyperplasia. Am J Clin Pathol. 1978;69(1):86-90. - PubMed
-
- Lachant NA, Sun NC, Leong LA, Oseas RS, Prince HE. Multicentric angiofollicular lymph node hyperplasia (Castleman’s disease) followed by Kaposi’s sarcoma in two homosexual males with the acquired immunodeficiency syndrome (AIDS). Am J Clin Pathol. 1985;83(1):27-33. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

