Protocells and RNA Self-Replication
- PMID: 30181195
- PMCID: PMC6120706
- DOI: 10.1101/cshperspect.a034801
Protocells and RNA Self-Replication
Abstract
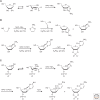
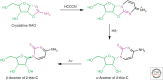
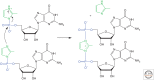
The general notion of an "RNA world" is that, in the early development of life on the Earth, genetic continuity was assured by the replication of RNA, and RNA molecules were the chief agents of catalytic function. Assuming that all of the components of RNA were available in some prebiotic locale, these components could have assembled into activated nucleotides that condensed to form RNA polymers, setting the stage for the chemical replication of polynucleotides through RNA-templated RNA polymerization. If a sufficient diversity of RNAs could be copied with reasonable rate and fidelity, then Darwinian evolution would begin with RNAs that facilitated their own reproduction enjoying a selective advantage. The concept of a "protocell" refers to a compartment where replication of the primitive genetic material took place and where primitive catalysts gave rise to products that accumulated locally for the benefit of the replicating cellular entity. Replication of both the protocell and its encapsulated genetic material would have enabled natural selection to operate based on the differential fitness of competing cellular entities, ultimately giving rise to modern cellular life.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
-
- Anastasi C, Crowe MA, Sutherland JD. 2007. Two-step potentially prebiotic synthesis of α-d-cytidine-5′-phosphate from d-glyceraldehyde-3-phosphate. J Am Chem Soc 129: 24–25. - PubMed
-
- Aumiller WM Jr, Pir Cakmak F, Davis BW, Keating CD. 2016. RNA-based coacervates as a model for membraneless organelles: Formation, properties, and interfacial liposome assembly. Langmuir 32: 10042–10053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
