Removal of a Membrane Anchor Reveals the Opposing Regulatory Functions of Vibrio cholerae Glucose-Specific Enzyme IIA in Biofilms and the Mammalian Intestine
- PMID: 30181246
- PMCID: PMC6123446
- DOI: 10.1128/mBio.00858-18
Removal of a Membrane Anchor Reveals the Opposing Regulatory Functions of Vibrio cholerae Glucose-Specific Enzyme IIA in Biofilms and the Mammalian Intestine
Abstract
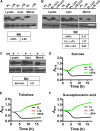
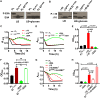
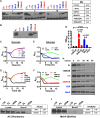
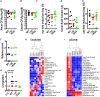
The Vibrio cholerae phosphoenolpyruvate phosphotransferase system (PTS) is a well-conserved, multicomponent phosphotransfer cascade that coordinates the bacterial response to carbohydrate availability through direct interactions of its components with protein targets. One such component, glucose-specific enzyme IIA (EIIAGlc), is a master regulator that coordinates bacterial metabolism, nutrient uptake, and behavior by direct interactions with cytoplasmic and membrane-associated protein partners. Here, we show that an amphipathic helix (AH) at the N terminus of V. cholerae EIIAGlc serves as a membrane association domain that is dispensable for interactions with cytoplasmic partners but essential for regulation of integral membrane protein partners. By deleting this AH, we reveal previously unappreciated opposing regulatory functions for EIIAGlc at the membrane and in the cytoplasm and show that these opposing functions are active in the laboratory biofilm and the mammalian intestine. Phosphotransfer through the PTS proceeds in the absence of the EIIAGlc AH, while PTS-dependent sugar transport is blocked. This demonstrates that the AH couples phosphotransfer to sugar transport and refutes the paradigm of EIIAGlc as a simple phosphotransfer component in PTS-dependent transport. Our findings show that Vibrio cholerae EIIAGlc, a central regulator of pathogen metabolism, contributes to optimization of bacterial physiology by integrating metabolic cues arising from the cytoplasm with nutritional cues arising from the environment. Because pathogen carbon metabolism alters the intestinal environment, we propose that it may be manipulated to minimize the metabolic cost of intestinal infection.IMPORTANCE The V. cholerae phosphoenolpyruvate phosphotransferase system (PTS) is a well-conserved, multicomponent phosphotransfer cascade that regulates cellular physiology and virulence in response to nutritional signals. Glucose-specific enzyme IIA (EIIAGlc), a component of the PTS, is a master regulator that coordinates bacterial metabolism, nutrient uptake, and behavior by direct interactions with protein partners. We show that an amphipathic helix (AH) at the N terminus of V. cholerae EIIAGlc serves as a membrane association domain that is dispensable for interactions with cytoplasmic partners but essential for regulation of integral membrane protein partners. By removing this amphipathic helix, hidden, opposing roles for cytoplasmic partners of EIIAGlc in both biofilm formation and metabolism within the mammalian intestine are revealed. This study defines a novel paradigm for AH function in integrating opposing regulatory functions in the cytoplasm and at the bacterial cell membrane and highlights the PTS as a target for metabolic modulation of the intestinal environment.
Keywords: Vibrio cholerae; amphipathic helix; biofilms; phosphoenolpyruvate phosphotransferase system; sugar transport.
Copyright © 2018 Vijayakumar et al.
Figures

Similar articles
-
Glucose-specific enzyme IIA has unique binding partners in the vibrio cholerae biofilm.mBio. 2012 Nov 6;3(6):e00228-12. doi: 10.1128/mBio.00228-12. mBio. 2012. PMID: 23131828 Free PMC article.
-
The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways.J Bacteriol. 2010 Jun;192(12):3055-67. doi: 10.1128/JB.00213-10. Epub 2010 Apr 16. J Bacteriol. 2010. PMID: 20400550 Free PMC article.
-
Glucose-Specific Enzyme IIA of the Phosphoenolpyruvate:Carbohydrate Phosphotransferase System Modulates Chitin Signaling Pathways in Vibrio cholerae.J Bacteriol. 2017 Aug 22;199(18):e00127-17. doi: 10.1128/JB.00127-17. Print 2017 Sep 15. J Bacteriol. 2017. PMID: 28461445 Free PMC article.
-
Transcription regulators controlled by interaction with enzyme IIB components of the phosphoenolpyruvate: sugar phosphotransferase system.Biochim Biophys Acta. 2013 Jul;1834(7):1415-24. doi: 10.1016/j.bbapap.2013.01.004. Epub 2013 Jan 11. Biochim Biophys Acta. 2013. PMID: 23318733 Review.
-
Carbohydrate Transport by Group Translocation: The Bacterial Phosphoenolpyruvate: Sugar Phosphotransferase System.Subcell Biochem. 2019;92:223-274. doi: 10.1007/978-3-030-18768-2_8. Subcell Biochem. 2019. PMID: 31214989 Review.
Cited by
-
Posttranscription Initiation Control of Gene Expression Mediated by Bacterial RNA-Binding Proteins.Annu Rev Microbiol. 2019 Sep 8;73:43-67. doi: 10.1146/annurev-micro-020518-115907. Epub 2019 May 17. Annu Rev Microbiol. 2019. PMID: 31100987 Free PMC article. Review.
-
Methionine Availability in the Arthropod Intestine Is Elucidated through Identification of Vibrio cholerae Methionine Acquisition Systems.Appl Environ Microbiol. 2020 May 19;86(11):e00371-20. doi: 10.1128/AEM.00371-20. Print 2020 May 19. Appl Environ Microbiol. 2020. PMID: 32220836 Free PMC article.
-
Diverse Mechanisms and Circuitry for Global Regulation by the RNA-Binding Protein CsrA.Front Microbiol. 2020 Oct 27;11:601352. doi: 10.3389/fmicb.2020.601352. eCollection 2020. Front Microbiol. 2020. PMID: 33193284 Free PMC article. Review.
-
Proteome and Physiological Characterization of Halotolerant Nodule Endophytes: The Case of Rahnella aquatilis and Serratia plymuthica.Microorganisms. 2022 Apr 24;10(5):890. doi: 10.3390/microorganisms10050890. Microorganisms. 2022. PMID: 35630335 Free PMC article.
-
Sequestration of a dual function DNA-binding protein by Vibrio cholerae CRP.Proc Natl Acad Sci U S A. 2022 Nov 16;119(46):e2210115119. doi: 10.1073/pnas.2210115119. Epub 2022 Nov 7. Proc Natl Acad Sci U S A. 2022. PMID: 36343262 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

