VPS13: A lipid transfer protein making contacts at multiple cellular locations
- PMID: 30181317
- PMCID: PMC6168276
- DOI: 10.1083/jcb.201808151
VPS13: A lipid transfer protein making contacts at multiple cellular locations
Abstract
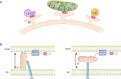
The evolutionarily conserved VPS13 proteins localize to multiple membrane contact sites though their function and regulation has been elusive. Bean et al. (2018. J. Cell Biol. https://doi.org/10.1083/jcb.201804111) found that competitive adaptors control the different localizations of yeast Vps13p, while Kumar et al. (2018. J. Cell Biol. https://doi.org/10.1083/jcb.201807019) provide biochemical and structural evidence for VPS13 proteins in the nonvesicular transport of phospholipids.
© 2018 Gao and Yang.
Figures
Comment on
-
Competitive organelle-specific adaptors recruit Vps13 to membrane contact sites.J Cell Biol. 2018 Oct 1;217(10):3593-3607. doi: 10.1083/jcb.201804111. Epub 2018 Jul 17. J Cell Biol. 2018. PMID: 30018089 Free PMC article.
-
VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites.J Cell Biol. 2018 Oct 1;217(10):3625-3639. doi: 10.1083/jcb.201807019. Epub 2018 Aug 9. J Cell Biol. 2018. PMID: 30093493 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

