Strategies to Address Chimeric Antigen Receptor Tonic Signaling
- PMID: 30181329
- PMCID: PMC6130819
- DOI: 10.1158/1535-7163.MCT-17-1097
Strategies to Address Chimeric Antigen Receptor Tonic Signaling
Abstract
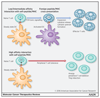
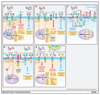
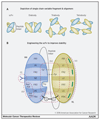
Adoptive cell transfer using chimeric antigen receptors (CAR) has emerged as one of the most promising new therapeutic modalities for patients with relapsed or refractory B-cell malignancies. Thus far, results in patients with advanced solid tumors have proven disappointing. Constitutive tonic signaling in the absence of ligand is an increasingly recognized complication when deploying these synthetic fusion receptors and can be a cause of poor antitumor efficacy, impaired survival, and reduced persistence in vivo In parallel, ligand-dependent tonic signaling can mediate toxicity and promote T-cell anergy, exhaustion, and activation-induced cell death. Here, we review the mechanisms underpinning CAR tonic signaling and highlight the wide variety of effects that can emerge after making subtle structural changes or altering the methodology of CAR transduction. We highlight strategies to prevent unconstrained tonic signaling and address its deleterious consequences. We also frame this phenomenon in the context of endogenous TCR tonic signaling, which has been shown to regulate peripheral tolerance, facilitate the targeting of foreign antigens, and suggest opportunities to coopt ligand-dependent CAR tonic signaling to facilitate in vivo persistence and efficacy. Mol Cancer Ther; 17(9); 1795-815. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
JM is chief scientific officer of Leucid Bio, a spinout company focussed on CAR T-cell and gamma delta T-cell immunotherapies for malignant disease. AA does not have any conflicts of interest to declare.
Figures


References
-
- Maher J. Clinical immunotherapy of B-cell malignancy using CD19-targeted CAR T-cells. Curr Gene Ther. 2014;14:35–43. - PubMed
-
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. - PMC - PubMed
-
- United States Food and Drug Administration Approval Letter 30th August 2017 - Kymriah. 2017.
-
- US regulator signs off on new $475,000 cancer therapy. 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

