Papillomaviruses and Endocytic Trafficking
- PMID: 30181457
- PMCID: PMC6163501
- DOI: 10.3390/ijms19092619
Papillomaviruses and Endocytic Trafficking
Abstract
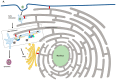
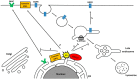
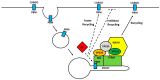
Endocytic trafficking plays a major role in transport of incoming human papillomavirus (HPVs) from plasma membrane to the trans Golgi network (TGN) and ultimately into the nucleus. During this infectious entry, several cellular sorting factors are recruited by the viral capsid protein L2, which plays a critical role in ensuring successful transport of the L2/viral DNA complex to the nucleus. Later in the infection cycle, two viral oncoproteins, E5 and E6, have also been shown to modulate different aspects of endocytic transport pathways. In this review, we highlight how HPV makes use of and perturbs normal endocytic transport pathways, firstly to achieve infectious virus entry, secondly to produce productive infection and the completion of the viral life cycle and, finally, on rare occasions, to bring about the development of malignancy.
Keywords: HPV; endocytic machinery; retriever; retromer; sorting nexins; viral capsid proteins; viral oncoproteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Rab6a enables BICD2/dynein-mediated trafficking of human papillomavirus from the trans-Golgi network during virus entry.mBio. 2024 Nov 13;15(11):e0281124. doi: 10.1128/mbio.02811-24. Epub 2024 Oct 21. mBio. 2024. PMID: 39431827 Free PMC article.
-
Human Papillomavirus 16 Infection Induces VAP-Dependent Endosomal Tubulation.J Virol. 2018 Feb 26;92(6):e01514-17. doi: 10.1128/JVI.01514-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29321327 Free PMC article.
-
A Novel PDZ Domain Interaction Mediates the Binding between Human Papillomavirus 16 L2 and Sorting Nexin 27 and Modulates Virion Trafficking.J Virol. 2015 Oct;89(20):10145-55. doi: 10.1128/JVI.01499-15. Epub 2015 Jul 22. J Virol. 2015. PMID: 26202251 Free PMC article.
-
Cruising the cellular highways: How human papillomavirus travels from the surface to the nucleus.Virus Res. 2017 Mar 2;231:1-9. doi: 10.1016/j.virusres.2016.10.015. Epub 2016 Oct 29. Virus Res. 2017. PMID: 27984059 Free PMC article. Review.
-
Subcellular Trafficking of the Papillomavirus Genome during Initial Infection: The Remarkable Abilities of Minor Capsid Protein L2.Viruses. 2017 Dec 3;9(12):370. doi: 10.3390/v9120370. Viruses. 2017. PMID: 29207511 Free PMC article. Review.
Cited by
-
Human Papillomavirus infection requires the CCT Chaperonin Complex.J Virol. 2021 May 10;95(11):e01943-20. doi: 10.1128/JVI.01943-20. Epub 2021 Mar 17. J Virol. 2021. PMID: 33731457 Free PMC article.
-
Molecular aspects of cervical cancer: a pathogenesis update.Front Oncol. 2024 Mar 19;14:1356581. doi: 10.3389/fonc.2024.1356581. eCollection 2024. Front Oncol. 2024. PMID: 38567159 Free PMC article. Review.
-
Rab6a enables BICD2/dynein-mediated trafficking of human papillomavirus from the trans-Golgi network during virus entry.mBio. 2024 Nov 13;15(11):e0281124. doi: 10.1128/mbio.02811-24. Epub 2024 Oct 21. mBio. 2024. PMID: 39431827 Free PMC article.
-
Role of Viral Ribonucleoproteins in Human Papillomavirus Type 16 Gene Expression.Viruses. 2020 Sep 30;12(10):1110. doi: 10.3390/v12101110. Viruses. 2020. PMID: 33007936 Free PMC article. Review.
-
Papillomaviruses Go Retro.Pathogens. 2020 Apr 7;9(4):267. doi: 10.3390/pathogens9040267. Pathogens. 2020. PMID: 32272661 Free PMC article. Review.
References
-
- Culp T.D., Budgeon L.R., Marinkovich M.P., Meneguzzi G., Christensen N.D. Keratinocyte-secreted laminin 5 can function as a transient receptor for human papillomaviruses by binding virions and transferring them to adjacent cells. J. Virol. 2006;80:8940–8950. doi: 10.1128/JVI.00724-06. - DOI - PMC - PubMed
-
- Combita A.L., Touzé A., Bousarghin L., Sizaret P.Y., Muñoz N., Coursaget P. Gene transfer using human papillomavirus pseudovirions varies according to virus genotype and requires cell surface heparan sulfate. FEMS Microbiol. Lett. 2001;204:183–188. doi: 10.1111/j.1574-6968.2001.tb10883.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

