Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View
- PMID: 30181463
- PMCID: PMC6164756
- DOI: 10.3390/bioengineering5030071
Understanding the Impact of Stent and Scaffold Material and Strut Design on Coronary Artery Thrombosis from the Basic and Clinical Points of View
Abstract
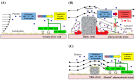
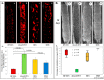
The technology of percutaneous coronary intervention (PCI) is constantly being refined in order to overcome the shortcomings of present day technologies. Even though current generation metallic drug-eluting stents (DES) perform very well in the short-term, concerns still exist about their long-term efficacy. Late clinical complications including late stent thrombosis (ST), restenosis, and neoatherosclerosis still exist and many of these events may be attributed to either the metallic platform and/or the drug and polymer left behind in the arterial wall. To overcome this limitation, the concept of totally bioresorbable vascular scaffolds (BRS) was invented with the idea that by eliminating long-term exposure of the vessel wall to the metal backbone, drug, and polymer, late outcomes would improve. The Absorb-bioabsorbable vascular scaffold (Absorb-BVS) represented the most advanced attempt to make such a device, with thicker struts, greater vessel surface area coverage and less radial force versus contemporary DES. Unfortunately, almost one year after its initial approval by the U.S. Food and Drug Administration, this scaffold was withdrawn from the market due to declining devise utilization driven by the concerns about scaffold thrombosis (ScT) seen in both early and late time points. Additionally, the specific causes of ScT have not yet been fully elucidated. In this review, we discuss the platform, vascular response, and clinical data of past and current metallic coronary stents with the Absorb-BVS and newer generation BRS, concentrating on their material/design and the mechanisms of thrombotic complications from the pre-clinical, pathologic, and clinical viewpoints.
Keywords: bioresorbable vascular scaffold; drug eluting stent; polymer; scaffold thrombosis; stent thrombosis.
Conflict of interest statement
Renu Virmani, has received honoraria from 480 Biomedical, Abbott Vascular, Boston Scientific, Cook Medical, Lutonix, Medtronic, Terumo Corporation and W.L. Gore; and is a consultant for 480 Biomedical, Abbott Vascular, Medtronic, and W.L. Gore. Aloke V. Finn, has sponsored research agreements with Boston Scientific and Medtronic CardioVascular, and is an advisory board member to Medtronic CardioVascular. All other authors declare no conflict of interest.
Figures



References
-
- Serruys P.W., de Jaegere P., Kiemeneij F., Macaya C., Rutsch W., Heyndrickx G., Emanuelsson H., Marco J., Legrand V., Materne P., et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. - DOI - PubMed
-
- Fischman D.L., Leon M.B., Baim D.S., Schatz R.A., Savage M.P., Penn I., Detre K., Veltri L., Ricci D., Nobuyoshi M., et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

