Cariprazine, A Dopamine D₂/D₃ Receptor Partial Agonist, Modulates ABCG2-Mediated Multidrug Resistance in Cancer
- PMID: 30181510
- PMCID: PMC6162716
- DOI: 10.3390/cancers10090308
Cariprazine, A Dopamine D₂/D₃ Receptor Partial Agonist, Modulates ABCG2-Mediated Multidrug Resistance in Cancer
Abstract
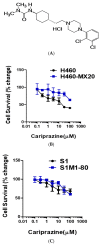
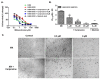
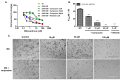
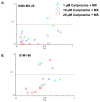
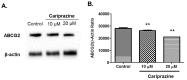
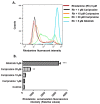
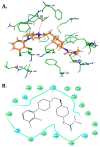
Multidrug resistance (MDR) is a continuing clinical problem that limits the efficacy of chemotherapy in cancer. The over expression of the ATP-binding cassette (ABC) family G2 (ABCG2) transporter is one of the main mechanisms that mediates MDR in cancer. Molecular modeling data indicated that cariprazine, a dopamine D₂/D₃ receptor partial agonist, had a significant binding affinity for ABCG2 transporter with a Glide XP score of -6.515. Therefore, in this in vitro study, we determined the effect of cariprazine on MDR resulting from the overexpression of ABCG2 transporters. Alone, cariprazine, at concentrations up to 20 μM, did not significantly decrease cell viability. Cariprazine, at concentrations ranging from 1 to 10 μM, did not significantly alter the cytotoxicity of mitoxantrone (MX) in the parental non-small cell cancer cell line, H460 and colon cancer cell S1. However, cariprazine (1⁻20 μM) significantly enhanced the efficacy of ABCG2 substrate antineoplastic drug MX in the ABCG2-overexpressing MDR cell line, H460-MX20 and S1M1-80, by reducing the resistance fold from 28 to 1 and from 93 to 1.33, respectively. Cariprazine, in a concentration-dependent (1⁻20 μM), significantly increased the intracellular accumulation of Rhodamine 123 in S1M1-80. Interestingly, 10 or 20 μM of cariprazine significantly decreased the expression levels of the ABCG2 protein in the colon and lung cancer cell lines, suggesting that cariprazine inhibits both the function and expression of ABCG2 transporters at nontoxic concentrations. Overall, our results suggest that cariprazine, via several distinct mechanisms, can resensitize resistant cancer cells to mitoxantrone.
Keywords: ABCG2; Cariprazine; Colon cancer; Dopamine D3-preferring D2/D3 receptor partial; Lung cancer; Multidrug resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The dopamine D3 receptor antagonists PG01037, NGB2904, SB277011A, and U99194 reverse ABCG2 transporter-mediated drug resistance in cancer cell lines.Cancer Lett. 2017 Jun 28;396:167-180. doi: 10.1016/j.canlet.2017.03.015. Epub 2017 Mar 18. Cancer Lett. 2017. PMID: 28323029
-
The AKT inhibitor, MK-2206, attenuates ABCG2-mediated drug resistance in lung and colon cancer cells.Front Pharmacol. 2023 Jul 13;14:1235285. doi: 10.3389/fphar.2023.1235285. eCollection 2023. Front Pharmacol. 2023. PMID: 37521473 Free PMC article.
-
Noninvasive Evaluation of Multidrug Resistance via Imaging of ABCG2/BCRP Multidrug Transporter in Lung Cancer Xenograft Models.Mol Pharm. 2022 Oct 3;19(10):3521-3529. doi: 10.1021/acs.molpharmaceut.1c00939. Epub 2022 Apr 15. Mol Pharm. 2022. PMID: 35427142
-
Dacomitinib potentiates the efficacy of conventional chemotherapeutic agents via inhibiting the drug efflux function of ABCG2 in vitro and in vivo.J Exp Clin Cancer Res. 2018 Feb 20;37(1):31. doi: 10.1186/s13046-018-0690-x. J Exp Clin Cancer Res. 2018. PMID: 29458405 Free PMC article.
-
Cariprazine for the Treatment of Schizophrenia: A Review of this Dopamine D3-Preferring D3/D2 Receptor Partial Agonist.Clin Schizophr Relat Psychoses. 2016 Summer;10(2):109-19. doi: 10.3371/1935-1232-10.2.109. Clin Schizophr Relat Psychoses. 2016. PMID: 27440212 Review.
Cited by
-
Microfluidic chip enables single-cell measurement for multidrug resistance in triple-negative breast cancer cells.Cancer Drug Resist. 2020 Mar 11;3(3):613-622. doi: 10.20517/cdr.2019.77. eCollection 2020. Cancer Drug Resist. 2020. PMID: 35582454 Free PMC article.
-
Cancer and the Dopamine D2 Receptor: A Pharmacological Perspective.J Pharmacol Exp Ther. 2019 Jul;370(1):111-126. doi: 10.1124/jpet.119.256818. Epub 2019 Apr 18. J Pharmacol Exp Ther. 2019. PMID: 31000578 Free PMC article. Review.
-
Newer antipsychotics: Brexpiprazole, cariprazine, and lumateperone: A pledge or another unkept promise?World J Psychiatry. 2021 Dec 19;11(12):1228-1238. doi: 10.5498/wjp.v11.i12.1228. eCollection 2021 Dec 19. World J Psychiatry. 2021. PMID: 35070772 Free PMC article. Review.
-
Repurposing Antipsychotics for Cancer Treatment.Biomedicines. 2021 Nov 28;9(12):1785. doi: 10.3390/biomedicines9121785. Biomedicines. 2021. PMID: 34944601 Free PMC article. Review.
-
Novel 3-((2-chloroquinolin-3-yl)methylene)indolin-2-one derivatives produce anticancer efficacy in ovarian cancer in vitro.Heliyon. 2019 May 14;5(5):e01603. doi: 10.1016/j.heliyon.2019.e01603. eCollection 2019 May. Heliyon. 2019. PMID: 31193218 Free PMC article.
References
-
- Gillet J.P., Gottesman M.M. Multi-Drug Resistance in Cancer. Humana Press; New York, NY USA: 2010. Mechanisms of multidrug resistance in Cancer; pp. 47–76. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

