Gold Catalyzed Multicomponent Reactions beyond A³ Coupling
- PMID: 30181514
- PMCID: PMC6225195
- DOI: 10.3390/molecules23092255
Gold Catalyzed Multicomponent Reactions beyond A³ Coupling
Abstract
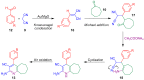
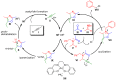
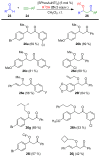
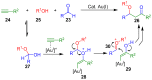
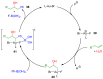
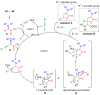
The preparation of complex architectures has inspired the search for new methods and new processes in organic synthesis. Multicomponent reactions have become an interesting approach to achieve such molecular diversity and complexity. This review intends to illustrate important gold-catalyzed examples for the past ten years leading to interesting skeletons involved in biologically active compounds.
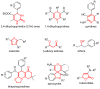
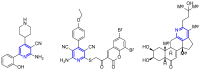
Keywords: 1,4-dihydropyridines; 3,4-dihydropyrimidin-2(1H)-ones; butenolides; catalysis; ethers; gold; multicomponent reactions; oxazoles; pyridines; spirocycles; thiazolo-quinolines; β-alkoxyketones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

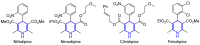











References
-
- Zhu J., Bienaymé H., editors. Multicomponent Reactions. Wiley-VCH; Weinheim, Germany: 2005.
-
- Herrera R.P., Marqués-López E., editors. Multicomponent Reactions: Concepts and Applications for Design and Synthesis. John Wiley & Sons; Hoboken, NJ, USA: 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

