Identification of a dioxin-responsive oxylipin signature in roots of date palm: involvement of a 9-hydroperoxide fatty acid reductase, caleosin/peroxygenase PdPXG2
- PMID: 30181584
- PMCID: PMC6123484
- DOI: 10.1038/s41598-018-31342-4
Identification of a dioxin-responsive oxylipin signature in roots of date palm: involvement of a 9-hydroperoxide fatty acid reductase, caleosin/peroxygenase PdPXG2
Abstract
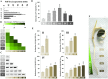
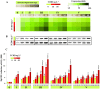
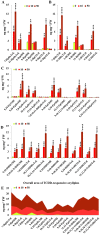
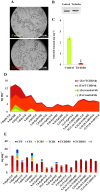
Dioxins are highly hazardous pollutants that have well characterized impacts on both animal and human health. However, the biological effects of dioxins on plants have yet to be described in detail. Here we describe a dioxin-inducible caleosin/peroxygenase isoform, PdPXG2, that is mainly expressed in the apical zone of date palm roots and specifically reduces 9-hydroperoxide fatty acids. A characteristic spectrum of 18 dioxin-responsive oxylipin (DROXYL) congeners was also detected in date palm roots after exposure to dioxin. Of particular interest, six oxylipins, mostly hydroxy fatty acids, were exclusively formed in response to TCDD. The DROXYL signature was evaluated in planta and validated in vitro using a specific inhibitor of PdPXG2 in a root-protoplast system. Comparative analysis of root suberin showed that levels of certain monomers, especially the mono-epoxides and tri-hydroxides of C16:3 and C18:3, were significantly increased after exposure to TCDD. Specific inhibition of PdPXG2 activity revealed a positive linear relationship between deposition of suberin in roots and their permeability to TCDD. The results highlight the involvement of this peroxygenase in the plant response to dioxin and suggest the use of dioxin-responsive oxylipin signatures as biomarkers for plant exposure to this important class of xenobiotic contaminants.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- WHO. Dioxins and their effects on human health. http://www.who.int/mediacentre/factsheets/fs225/en/, Updated October 2016 (2016).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

