Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth
- PMID: 30181702
- PMCID: PMC6115345
- DOI: 10.4196/kjpp.2018.22.5.555
Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth
Abstract
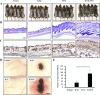
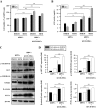
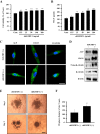
Human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) are used in tissue repair and regeneration; however, the mechanisms involved are not well understood. We investigated the hair growth-promoting effects of hUCB-MSCs treatment to determine whether hUCB-MSCs enhance the promotion of hair growth. Furthermore, we attempted to identify the factors responsible for hair growth. The effects of hUCB-MSCs on hair growth were investigated in vivo, and hUCB-MSCs advanced anagen onset and hair follicle neogeneration. We found that hUCB-MSCs co-culture increased the viability and up-regulated hair induction-related proteins of human dermal papilla cells (hDPCs) in vitro. A growth factor antibody array revealed that secretory factors from hUCB-MSCs are related to hair growth. Insulin-like growth factor binding protein-1 (IGFBP-1) and vascular endothelial growth factor (VEGF) were increased in co-culture medium. Finally, we found that IGFBP-1, through the co-localization of an IGF-1 and IGFBP-1, had positive effects on cell viability; VEGF secretion; expression of alkaline phosphatase (ALP), CD133, and β-catenin; and formation of hDPCs 3D spheroids. Taken together, these data suggest that hUCB-MSCs promote hair growth via a paracrine mechanism.
Keywords: Alopecia; Dermal papilla cell; Hair growth; IGFBP-1; Stem cell; Stem-cell therapy.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
Figures


References
-
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. - PubMed
-
- Harada T, Izaki S, Tsutsumi H, Kobayashi M, Kitamura K. Apoptosis of hair follicle cells in the second-degree burn wound unders hypernatremic conditions. Burns. 1998;24:464–469. - PubMed
-
- Leyden J, Dunlap F, Miller B, Winters P, Lebwohl M, Hecker D, Kraus S, Baldwin H, Shalita A, Draelos Z, Markou M, Thiboutot D, Rapaport M, Kang S, Kelly T, Pariser D, Webster G, Hordinsky M, Rietschel R, Katz HI, Terranella L, Best S, Round E, Waldstreicher J. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999;40:930–937. - PubMed
-
- Kligman AM. Pathologic dynamics of human hair loss. I. Telogen effuvium. Arch Dermatol. 1961;83:175–198. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

