Structure of graphene and its disorders: a review
- PMID: 30181789
- PMCID: PMC6116708
- DOI: 10.1080/14686996.2018.1494493
Structure of graphene and its disorders: a review
Abstract
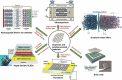
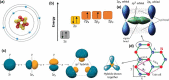
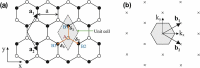
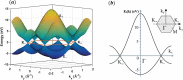
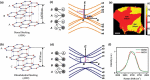
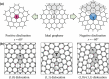
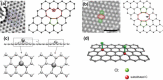
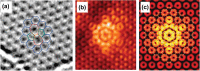
Monolayer graphene exhibits extraordinary properties owing to the unique, regular arrangement of atoms in it. However, graphene is usually modified for specific applications, which introduces disorder. This article presents details of graphene structure, including sp2 hybridization, critical parameters of the unit cell, formation of σ and π bonds, electronic band structure, edge orientations, and the number and stacking order of graphene layers. We also discuss topics related to the creation and configuration of disorders in graphene, such as corrugations, topological defects, vacancies, adatoms and sp3-defects. The effects of these disorders on the electrical, thermal, chemical and mechanical properties of graphene are analyzed subsequently. Finally, we review previous work on the modulation of structural defects in graphene for specific applications.
Keywords: 10 Engineering and Structural materials; 104 Carbon and related materials; 105 Low-Dimension (1D/2D) materials; 2D materials; 302 Crystallization / Heat treatment / Crystal growth; Graphene; defects modulation; disorder; review; structure.
Figures












References
-
- Wallace PR. The band theory of graphite. Phys Rev. 1947;71:622–634.
-
- Fradkin E. Critical behavior of disordered degenerate semiconductors. II. Spectrum and transport properties in mean-field theory. Phys Rev B. 1986;33:3263–3268. - PubMed
-
- Mermin ND. Crystalline order in two dimensions. Phys Rev. 1968;176:250–254.
-
- Land TA, Michely T, Behm RJ, et al. STM investigation of single layer graphite structures produced on Pt(111) by hydrocarbon decomposition. Surf Sci. 1992;264:261–270.
-
- Ohashi Y, Koizumi T, Yoshikawa T, et al. Size effect in the in-plane electrical resistivity of very thin graphite crystals. Tanso. 1997;1997:235–238.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
