Biomimetic delivery of signals for bone tissue engineering
- PMID: 30181921
- PMCID: PMC6115422
- DOI: 10.1038/s41413-018-0025-8
Biomimetic delivery of signals for bone tissue engineering
Abstract
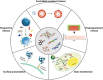
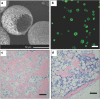
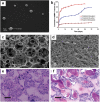
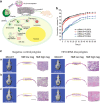
Bone tissue engineering is an exciting approach to directly repair bone defects or engineer bone tissue for transplantation. Biomaterials play a pivotal role in providing a template and extracellular environment to support regenerative cells and promote tissue regeneration. A variety of signaling cues have been identified to regulate cellular activity, tissue development, and the healing process. Numerous studies and trials have shown the promise of tissue engineering, but successful translations of bone tissue engineering research into clinical applications have been limited, due in part to a lack of optimal delivery systems for these signals. Biomedical engineers are therefore highly motivated to develop biomimetic drug delivery systems, which benefit from mimicking signaling molecule release or presentation by the native extracellular matrix during development or the natural healing process. Engineered biomimetic drug delivery systems aim to provide control over the location, timing, and release kinetics of the signal molecules according to the drug's physiochemical properties and specific biological mechanisms. This article reviews biomimetic strategies in signaling delivery for bone tissue engineering, with a focus on delivery systems rather than specific molecules. Both fundamental considerations and specific design strategies are discussed with examples of recent research progress, demonstrating the significance and potential of biomimetic delivery systems for bone tissue engineering.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Bone tissue engineering: a review in bone biomimetics and drug delivery strategies.Biotechnol Prog. 2009 Nov-Dec;25(6):1539-60. doi: 10.1002/btpr.246. Biotechnol Prog. 2009. PMID: 19824042 Review.
-
Advanced Polymer-Based Drug Delivery Strategies for Meniscal Regeneration.Tissue Eng Part B Rev. 2021 Jun;27(3):266-293. doi: 10.1089/ten.TEB.2020.0156. Epub 2020 Oct 27. Tissue Eng Part B Rev. 2021. PMID: 32988289 Review.
-
Biomimetic Approaches in Cardiac Tissue Engineering: Replicating the Native Heart Microenvironment.Cureus. 2023 Aug 13;15(8):e43431. doi: 10.7759/cureus.43431. eCollection 2023 Aug. Cureus. 2023. PMID: 37581196 Free PMC article. Review.
-
Oligoaniline-based conductive biomaterials for tissue engineering.Acta Biomater. 2018 May;72:16-34. doi: 10.1016/j.actbio.2018.03.042. Epub 2018 Apr 4. Acta Biomater. 2018. PMID: 29625254 Review.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
Cited by
-
The Interaction of Temozolomide with Blood Components Suggests the Potential Use of Human Serum Albumin as a Biomimetic Carrier for the Drug.Biomolecules. 2020 Jul 9;10(7):1015. doi: 10.3390/biom10071015. Biomolecules. 2020. PMID: 32659914 Free PMC article.
-
Microfluidic fabrication of microcarriers with sequential delivery of VEGF and BMP-2 for bone regeneration.Sci Rep. 2020 Jul 16;10(1):11764. doi: 10.1038/s41598-020-68221-w. Sci Rep. 2020. PMID: 32678204 Free PMC article.
-
Ciprofloxacin-Loaded Titanium Nanotubes Coated with Chitosan: A Promising Formulation with Sustained Release and Enhanced Antibacterial Properties.Pharmaceutics. 2022 Jun 27;14(7):1359. doi: 10.3390/pharmaceutics14071359. Pharmaceutics. 2022. PMID: 35890255 Free PMC article.
-
Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems.ACS Biomater Sci Eng. 2023 Sep 11;9(9):5222-5254. doi: 10.1021/acsbiomaterials.3c00609. Epub 2023 Aug 16. ACS Biomater Sci Eng. 2023. PMID: 37585562 Free PMC article. Review.
-
Efficacy of Three-Dimensional Bioactive Composites in Long Bone Repair with Photobiomodulation.Materials (Basel). 2025 Apr 9;18(8):1704. doi: 10.3390/ma18081704. Materials (Basel). 2025. PMID: 40333272 Free PMC article.
References
-
- Bone Grafts and Substitutes Market Analysis by Material (Natural—Autografts, Allografts; Synthetic—Ceramic, Composite, Polymer, Bone Morphogenetic Proteins (BMP)), By Application (Craniomaxillofacial, Dental, Foot & Ankle, Joint Reconstruction, Long Bone, Spinal Fusion) Forecasts to 2024, Bone Grafts and Substitutes Market Size, Share Report, 2024, 80 (2016).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

