Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing
- PMID: 30182056
- PMCID: PMC6118649
- DOI: 10.1126/sciadv.aas9459
Toward digitally controlled catalyst architectures: Hierarchical nanoporous gold via 3D printing
Abstract
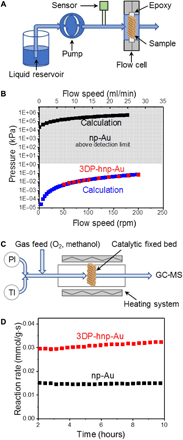
Monolithic nanoporous metals, derived from dealloying, have a unique bicontinuous solid/void structure that provides both large surface area and high electrical conductivity, making them ideal candidates for various energy applications. However, many of these applications would greatly benefit from the integration of an engineered hierarchical macroporous network structure that increases and directs mass transport. We report on 3D (three-dimensional)-printed hierarchical nanoporous gold (3DP-hnp-Au) with engineered nonrandom macroarchitectures by combining 3D printing and dealloying. The material exhibits three distinct structural length scales ranging from the digitally controlled macroporous network structure (10 to 1000 μm) to the nanoscale pore/ligament morphology (30 to 500 nm) controlled by dealloying. Supercapacitance, pressure drop, and catalysis measurements reveal that the 3D hierarchical nature of our printed nanoporous metals markedly improves mass transport and reaction rates for both liquids and gases. Our approach can be applied to a variety of alloy systems and has the potential to revolutionize the design of (electro-)chemical plants by changing the scaling relations between volume and catalyst surface area.
Figures




References
-
- Fujita T., Qian L.-H., Inoke K., Erlebacher J., Chen M.-W., Three-dimensional morphology of nanoporous gold. Appl. Phys. Lett. 92, 251902 (2008).
-
- Qi Z., Weissmüller J., Hierarchical nested-network nanostructure by dealloying. ACS Nano 7, 5948–5954 (2013). - PubMed
-
- Erlebacher J., Aziz M. J., Karma A., Dimitrov N., Sieradzki K., Evolution of nanoporosity in dealloying. Nature 410, 450–453 (2001). - PubMed
-
- A. Wittstock, J. Biener, J. Erlebacher, M. Baumer, Nanoporous Gold: From an Ancient Technology to a High-Tech Material (Royal Society of Chemistry, 2012).
-
- Wittstock A., Zielasek V., Biener J., Friend C. M., Bäumer M., Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 327, 319–322 (2010). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

