Review of clinical approaches in fluorescence lifetime imaging ophthalmoscopy
- PMID: 30182580
- PMCID: PMC8357196
- DOI: 10.1117/1.JBO.23.9.091415
Review of clinical approaches in fluorescence lifetime imaging ophthalmoscopy
Erratum in
-
Review of clinical approaches in fluorescence lifetime imaging ophthalmoscopy (Erratum).J Biomed Opt. 2018 Sep;23(9):1. doi: 10.1117/1.JBO.23.9.099802. J Biomed Opt. 2018. PMID: 30259716 Free PMC article.
Abstract
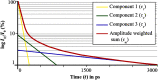
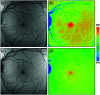
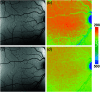
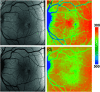
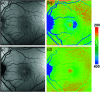
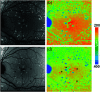
Autofluorescence-based imaging techniques have become very important in the ophthalmological field. Being noninvasive and very sensitive, they are broadly used in clinical routines. Conventional autofluorescence intensity imaging is largely influenced by the strong fluorescence of lipofuscin, a fluorophore that can be found at the level of the retinal pigment epithelium. However, different endogenous retinal fluorophores can be altered in various diseases. Fluorescence lifetime imaging ophthalmoscopy (FLIO) is an imaging modality to investigate the autofluorescence of the human fundus in vivo. It expands the level of information, as an addition to investigating the fluorescence intensity, and autofluorescence lifetimes are captured. The Heidelberg Engineering Spectralis-based fluorescence lifetime imaging ophthalmoscope is used to investigate a 30-deg retinal field centered at the fovea. It detects FAF decays in short [498 to 560 nm, short spectral channel (SSC) and long (560 to 720 nm, long spectral channel (LSC)] spectral channels, the mean fluorescence lifetimes (τm) are calculated using bi- or triexponential approaches. These are meant to be relatively independent of the fluorophore's intensity; therefore, fluorophores with less intense fluorescence can be detected. As an example, FLIO detects the fluorescence of macular pigment, retinal carotenoids that help protect the human fundus from light damages. Furthermore, FLIO is able to detect changes related to various retinal diseases, such as age-related macular degeneration, albinism, Alzheimer's disease, diabetic retinopathy, macular telangiectasia type 2, retinitis pigmentosa, and Stargardt disease. Some of these changes can already be found in healthy eyes and may indicate a risk to developing such diseases. Other changes in already affected eyes seem to indicate disease progression. This review article focuses on providing detailed information on the clinical findings of FLIO. This technique detects not only structural changes at very early stages but also metabolic and disease-related alterations. Therefore, it is a very promising tool that might soon be used for early diagnostics.
Keywords: fluorescence lifetime; fluorescence lifetime imaging ophthalmoscopy; lipofuscin; macular pigment; protein glycation; retinal disease; time-resolved fundus autofluorescence.
(2018) COPYRIGHT Society of Photo-Optical Instrumentation Engineers (SPIE).
Figures







References
-
- Lakowicz J., Principles of Fluorescence Spectroscopy, Plenum Publishers, New York: (1999).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

