Real-world benefits of allergen immunotherapy for birch pollen-associated allergic rhinitis and asthma
- PMID: 30183091
- PMCID: PMC6585786
- DOI: 10.1111/all.13598
Real-world benefits of allergen immunotherapy for birch pollen-associated allergic rhinitis and asthma
Abstract
Background: Real-world evidence is sparse on the benefits of allergen immunotherapy [AIT; subcutaneous/sublingual immunotherapy (SCIT/SLIT)], the only disease-modifying intervention for allergic rhinitis (AR) with long-term efficacy. This real-life study evaluated the effect of six AITs (native pollen SLIT/SCIT, four allergoid SCITs) vs symptomatic medication use, on AR symptoms and asthma symptoms/onset, in patients with birch pollen-associated AR and/or asthma.
Methods: In this retrospective cohort analysis of a German longitudinal prescription database, AIT patients received ≥2 successive seasonal treatment cycles; non-AIT patients had ≥3 AR prescriptions in three seasons or previous month. Patients were matched for: index year, age, gender, main indication at index, number of seasonal cycles within treatment period, baseline AR/asthma treatment prescriptions. Multiple regression analysis compared prescription data in AIT and non-AIT groups as proxy for clinical status/disease progression.
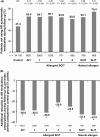
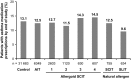
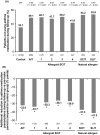
Results: Up to 6 years of follow-up, significantly more AIT (65.4%) vs non-AIT (47.4%) patients were AR medication-free; odds ratio (OR) [95% confidence interval (CI)]: 0.51 [(0.48-0.54); P < 0.001] (28.6% covariate-adjusted reduction vs non-AIT; P < 0.001), and significantly more AIT (49.1%) vs non-AIT (35.1%) patients were asthma medication-free [OR (95% CI): 0.59 (0.55-0.65); P < 0.001] (32% reduction vs non-AIT; P < 0.001), or reduced existing asthma medication use (32% covariate-adjusted reduction vs non-AIT; P < 0.001). During treatment, new-onset asthma risk was significantly reduced in the AIT vs non-AIT group (OR: 0.83; P = 0.001).
Conclusions: Birch pollen AIT demonstrated real-world benefits up to 6 years post-treatment cessation through significantly reduced AR and asthma medication intake, and significantly decreased risk of new-onset asthma medication use on-treatment.
Keywords: allergic rhinitis; asthma; real-world evidence; subcutaneous immunotherapy; sublingual immunotherapy.
© 2018 The Authors. Allergy Published by John Wiley & Sons Ltd.
Conflict of interest statement
U Wahn has received consulting fees from Allergopharma, Danone, Hipp, Merck, Novartis, IMS Health GmbH & Co and Stallergenes Greer; honoraria for lectures from ALK‐Abelló, Allergopharma, Allergy Therapeutics, LETI, MSD, Nestlé, Novartis, Nutricia and Stallergenes Greer; and research funding from Stallergenes Greer. C Bachert has received consulting fees or honoraria for lectures from ALK‐Abelló, Stallergenes Greer, HAL Allergy and IMS Health GmbH & Co. S Zielen has received fees for lectures and advisory boards from ALK‐Abelló Arzneimittel GmbH, Allergopharma GmbH, Allergy Therapeutics, Lofarma GmbH, bene‐Arzneimittel GmbH, Biotest, Boehringer Ingelheim, GlaxoSmithKline GmbH, IMS Health GmbH & Co. OHG, Novartis AG and Vifor Pharma Deutschland GmbH. J Heinrich has received consulting fees from Boehringer Ingelheim, IMS Health GmbH & Co. OHG and Kabi. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Canonica GW, Bousquet J, Mullol J, et al. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;62(Suppl 85):17‐25. - PubMed
-
- Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8:108‐352. - PubMed
-
- Global Atlas of Allergic Rhinitis and Chronic Rhinosinusitis . European Academy of Allergy and Clinical Immunology (EAACI), 2015. Available at: http://www.eaaci.org/globalatlas/ENT_Atlas_web.pdf. Accessed February 15, 2018
-
- Wahn U, Calderon MA, Demoly P. Real‐life clinical practice and management of polysensitized patients with respiratory allergies: a large, global survey of clinicians prescribing allergen immunotherapy. Expert Rev Clin Immunol. 2017;13:283‐289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

