Alirocumab safety in people with and without diabetes mellitus: pooled data from 14 ODYSSEY trials
- PMID: 30183102
- PMCID: PMC6585811
- DOI: 10.1111/dme.13817
Alirocumab safety in people with and without diabetes mellitus: pooled data from 14 ODYSSEY trials
Abstract
Aim: To evaluate the safety of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab according to diabetes mellitus status.
Methods: Safety data from 14 trials (8-104-week durations) were analysed by treatment (alirocumab or placebo/ezetimibe control) and diabetes status (yes/no, defined by medical history). Adverse event data were assessed using descriptive statistics and Cox models.
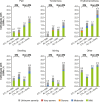
Results: Of the 5234 trial participants, 1554 (29.7%) had diabetes. Overall, treatment-emergent adverse events were similar in the alirocumab and control groups, except for more frequent local injection site reactions with alirocumab. Fewer people with diabetes experienced local injection site reactions [alirocumab, 3.5%, control, 2.9%; hazard ratio 1.24 (95% CI 0.68-2.25)] than those without diabetes [alirocumab, 7.5%; control, 4.9%; hazard ratio 1.51 (95% CI 1.13-2.01)]. Those with diabetes reported a greater number of serious adverse events (alirocumab, 19.4%; control, 19.7%) than those without diabetes (alirocumab, 14.5%; control, 13.5%). In people with diabetes, major adverse cardiac events occurred in 2.7% of alirocumab-treated people [control, 3.3%; hazard ratio 0.74 (95% CI 0.41-1.35)]; in those without diabetes, 1.8% of alirocumab-treated people had major adverse cardiac events [control, 1.7%; hazard ratio 0.95 (95% CI 0.56-1.62)]. Overall, no increase in HbA1c or fasting plasma glucose vs control treatment groups was observed, regardless of diabetes status.
Conclusion: This pooled analysis across 14 trials demonstrated similar safety for alirocumab vs control treatment, irrespective of diabetes status, except for more frequent local injection site reactions with alirocumab. People with diabetes reported fewer local injection site reactions than those without diabetes.
Trial registration: ClinicalTrials.gov NCT01288443 NCT01288469 NCT01266876 NCT01812707 NCT01507831 NCT01617655 NCT01644175 NCT01623115 NCT01709500 NCT01644188 NCT01730040 NCT01730053 NCT01644474 NCT01709513.
© 2018 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.
Figures



References
-
- Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16: 434–444. - PubMed
-
- Regeneron Pharmaceuticals, Inc . Regeneron and Sanofi Announce Plans to Make Praluent® (alirocumab) More Accessible and Affordable for Patients with the Greatest Health Risk and Unmet Need. Available at http://investor.regeneron.com/releasedetail.cfm?releaseid=1060465. Last accessed 29 March 2018.
-
- US Food and Drug Administration . Praluent prescribing information (US). Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s001lbl.pdf. Last accessed 7 August 2017.
-
- European Commission . Praluent summary of product characteristics (EC). Available at http://ec.europa.eu/health/documents/community-register/2015/20150923132.... Last accessed 9 Sep 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

