Thermodynamics of Iron(II) and Substrate Binding to the Ethylene-Forming Enzyme
- PMID: 30183265
- PMCID: PMC7199160
- DOI: 10.1021/acs.biochem.8b00730
Thermodynamics of Iron(II) and Substrate Binding to the Ethylene-Forming Enzyme
Abstract
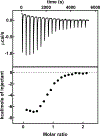
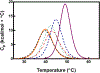
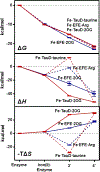
The ethylene-forming enzyme (EFE), like many other 2-oxoglutarate (2OG)-dependent nonheme iron(II) oxygenases, catalyzes the oxidative decarboxylation of 2OG to succinate and CO2 to generate a highly reactive iron species that hydroxylates a specific alkane C-H bond, in this case targeting l-arginine (Arg) for hydroxylation. However, the prominently observed reactivity of EFE is the transformation of 2OG into ethylene and three molecules of CO2. Crystallographic and biochemical studies have led to several proposed mechanisms for this 2-fold reactivity, but the detailed reaction steps are still obscure. Here, the thermodynamics associated with iron(II), 2OG, and Arg binding to EFE are studied using calorimetry (isothermal titration calorimetry and differential scanning calorimetry) to gain insight into how these binding equilibria organize the active site of EFE, which may have an impact on the O2 activation pathways observed in this system. Calorimetric data show that the addition of iron(II), Arg, and 2OG increases the stability over that of the apoenzyme, and there is distinctive cooperativity between substrate and cofactor binding. The energetics of binding of 2OG to Fe·EFE are consistent with a unique monodentate binding mode, which is different than the prototypical 2OG coordination mode in other 2OG-dependent oxygenases. This difference in the pre-O2 activation equilibria may be important for supporting the alternative ethylene-forming chemistry of EFE.
Conflict of interest statement
The authors declare no competing financial interest.
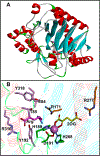
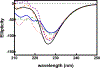
Figures
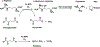

References
-
- Fernelius CW, Wittcoff H, and Varnerin RE (1979) Ethylene: The organic chemical industry’s most important building block. J. Chem. Educ 56, 385–387.
-
- Ghanta M, Fahey D, and Subramaniam B. (2014) Environmental impacts of ethylene production from diverse feed-stocks and energy sources. Appl. Petrochem. Res 4, 167–179.
-
- Bleecker AB, and Kende H. (2000) Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol 16, 1–18. - PubMed
-
- Dijkmans T, Pyl SP, Reyniers M-F, Abhari R, Van Geem KM, and Marin GB (2013) Production of bio-ethene and propene: alternatives for bulk chemicals and polymers. Green Chem. 15, 3064–3076.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

