The Effects of IFN-λ on Epithelial Barrier Function Contribute to Klebsiella pneumoniae ST258 Pneumonia
- PMID: 30183325
- PMCID: PMC6376406
- DOI: 10.1165/rcmb.2018-0021OC
The Effects of IFN-λ on Epithelial Barrier Function Contribute to Klebsiella pneumoniae ST258 Pneumonia
Abstract
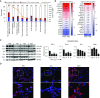
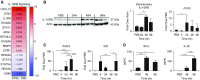
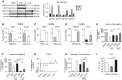
IFN-λ and IL-22, cytokines that share the coreceptor IL-10RB, are both induced over the course of Klebsiella pneumoniae ST258 (KP35) pneumonia. IL-22 is known to protect mucosal barriers, whereas the effects of IFN-λ on the mucosa are not established. We postulated that IFN-λ plays a role in regulating the airway epithelial barrier to facilitate cellular trafficking to the site of infection. In response to IFN-λ, the transmigration of neutrophils across a polarized monolayer of airway epithelial cells was increased, consistent with diminished epithelial integrity. KP35 infection increased epithelial permeability, and pretreatment with IFN-λ amplified this effect and facilitated bacterial transmigration. These effects of IFN-λ were confirmed in vivo, in that mice lacking the receptor for IFN-λ (Ifnlr1-/-) were protected from bacteremia in a murine model of KP35 pneumonia. Conversely, the integrity of the epithelial barrier was protected by IL-22, with subsequent impairment of neutrophil and bacterial transmigration in vitro. Maximal expression of IL-22 in vivo was observed later in the course of infection than IFN-λ production, with high levels of IL-22 produced by recruited immune cells at 48 hours, consistent with a role in epithelial barrier recovery. The divergent and opposing expression of these two related cytokines suggests a regulated interaction in the host response to KP35 infection. A major physiological effect of IFN-λ signaling is a decrease in epithelial barrier integrity, which facilitates immune cell recruitment but also enables K. pneumoniae invasion.
Keywords: IFN-λ; IL-22; airway epithelial barrier; bacterial pneumonia.
Figures


References
-
- Gomez-Simmonds A, Greenman M, Sullivan SB, Tanner JP, Sowash MG, Whittier S, et al. Population structure of Klebsiella pneumoniae causing bloodstream infections at a New York City tertiary care hospital: diversification of multidrug-resistant isolates. J Clin Microbiol. 2015;53:2060–2067. - PMC - PubMed
-
- Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, et al. ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70:2133–2143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

