New modalities of allergen immunotherapy
- PMID: 30183485
- PMCID: PMC6343630
- DOI: 10.1080/21645515.2018.1502126
New modalities of allergen immunotherapy
Abstract
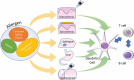
Allergen immunotherapy is a rapidly evolving field. Although subcutaneous immunotherapy has been practiced for over a hundred years, improved understanding of the underlying immunological mechanisms has led to the development of new, efficacious and better tolerated allergen-derivatives, adjuvants and encapsulated allergens. Diverse routes of allergen immunotherapy - oral, sublingual, epicutanoeus and intralymphatic - are enabling immunotherapy for anaphylactic food allergies and pollen-food allergy syndrome, while improving the tolerability and effectiveness of aeroallergen immunotherapy. The addition of Anti-IgE therapy decreases adverse effects of subcutaneous and oral immunotherapy.
Keywords: Allergen Immunotherapy; Anti-IgE; Epicutaneous Immunotherapy; Oral Immunotherapy; Subcutaneous Immunotherapy; Sublingual Immunotherapy; adjuvants.
Figures
Similar articles
-
One-year evaluation of clinical and immunological efficacy and safety of sublingual versus subcutaneous allergen immunotherapy in allergic conjunctivitis.Graefes Arch Clin Exp Ophthalmol. 2019 Sep;257(9):1989-1996. doi: 10.1007/s00417-019-04389-w. Epub 2019 Jun 17. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 31209565
-
Sublingual allergen immunotherapy: immunological mechanisms and prospects for refined vaccine preparation.Curr Med Chem. 2007;14(21):2235-44. doi: 10.2174/092986707781696609. Curr Med Chem. 2007. PMID: 17896972 Review.
-
[The route is the destination-novel forms of application of allergen immunotherapy].Hautarzt. 2021 Sep;72(9):776-783. doi: 10.1007/s00105-021-04869-3. Epub 2021 Aug 13. Hautarzt. 2021. PMID: 34387710 Review. German.
-
Peanut oral immunotherapy: current trends in clinical trials.Immunother Adv. 2022 Jan 31;2(1):ltac004. doi: 10.1093/immadv/ltac004. eCollection 2022. Immunother Adv. 2022. PMID: 35919493 Free PMC article. Review.
-
Oral and Sublingual Immunotherapy for Treatment of IgE-Mediated Food Allergy.Clin Rev Allergy Immunol. 2018 Oct;55(2):139-152. doi: 10.1007/s12016-018-8677-0. Clin Rev Allergy Immunol. 2018. PMID: 29656306 Review.
Cited by
-
Case Report: Safe and Effective Sublingual Birch Allergen Immunotherapy in Two HIV-Positive Patients.Front Immunol. 2021 Jul 27;12:599955. doi: 10.3389/fimmu.2021.599955. eCollection 2021. Front Immunol. 2021. PMID: 34385997 Free PMC article.
-
The Role of Regulatory T Cells in Epicutaneous Immunotherapy for Food Allergy.Front Immunol. 2021 Jul 8;12:660974. doi: 10.3389/fimmu.2021.660974. eCollection 2021. Front Immunol. 2021. PMID: 34305893 Free PMC article. Review.
-
Manufacturing processes of peanut (Arachis hypogaea) allergen powder-dnfp.Front Allergy. 2022 Oct 11;3:1004056. doi: 10.3389/falgy.2022.1004056. eCollection 2022. Front Allergy. 2022. PMID: 36304076 Free PMC article.
-
Current and Future Strategies for the Diagnosis and Treatment of the Alpha-Gal Syndrome (AGS).J Asthma Allergy. 2022 Jul 18;15:957-970. doi: 10.2147/JAA.S265660. eCollection 2022. J Asthma Allergy. 2022. PMID: 35879928 Free PMC article. Review.
-
Oral Tolerance Induction-Opportunities and Mechanisms.Foods. 2022 Oct 27;11(21):3386. doi: 10.3390/foods11213386. Foods. 2022. PMID: 36360000 Free PMC article. Review.
References
-
- Turkalj M, Banic I, Anzic SA.. A review of clinical efficacy, safety, new developments and adherence to allergen-specific immunotherapy in patients with allergic rhinitis caused by allergy to ragweed pollen (Ambrosia artemisiifolia). Patient Prefer Adherence. 2017;11:247–257. doi:10.2147/PPA.S70411. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
