Preclinical pharmacokinetics and pharmacodynamics of DCLL9718A: An antibody-drug conjugate for the treatment of acute myeloid leukemia
- PMID: 30183491
- PMCID: PMC6284592
- DOI: 10.1080/19420862.2018.1517565
Preclinical pharmacokinetics and pharmacodynamics of DCLL9718A: An antibody-drug conjugate for the treatment of acute myeloid leukemia
Abstract
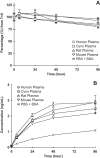
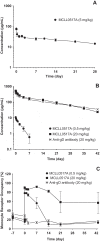
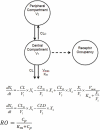
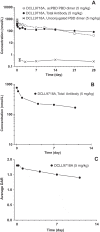
Few treatment options are available for acute myeloid leukemia (AML) patients. DCLL9718A is an antibody-drug conjugate that targets C-type lectin-like molecule-1 (CLL-1). This receptor is prevalent on monocytes, neutrophils, and AML blast cells, and unlike CD33, is not expressed on hematopoietic stem cells, thus providing possible hematopoietic recovery. DCLL9718A comprises an anti-CLL-1 IgG1 antibody (MCLL0517A) linked to a pyrrolobenzodiazepine (PBD) dimer payload, via a cleavable disulfide-labile linker. Here, we characterize the in vitro and in vivo stability, the pharmacokinetics (PK) and pharmacodynamics (PD) of DCLL9718A and MCLL0517A in rodents and cynomolgus monkeys. Three key PK analytes were measured in these studies: total antibody, antibody-conjugated PBD dimer and unconjugated PBD dimer. In vitro, DCLL9718A, was stable with most (> 80%) of the PBD dimer payload remaining conjugated to the antibody over 96 hours. This was recapitulated in vivo with antibody-conjugated PBD dimer clearance estimates similar to DCLL9718A total antibody clearance. Both DCLL9718A and MCLL0517A showed linear PK in the non-binding rodent species, and non-linear PK in cynomolgus monkeys, a binding species. The PK data indicated minimal impact of conjugation on the disposition of DCLL9718A total antibody. Finally, in cynomolgus monkey, MCLL0517A showed target engagement at all doses tested (0.5 and 20 mg/kg) as measured by receptor occupancy, and DCLL9718A (at doses of 0.05, 0.1 and 0.2 mg/kg) showed strong PD activity as evidenced by notable reduction in monocytes and neutrophils.
Keywords: Antibody-drug conjugate; CLL-1; PBD dimer; acute myeloid leukemia; pharmacokinetics; receptor occupancy.
Figures
Similar articles
-
An Anti-CLL-1 Antibody-Drug Conjugate for the Treatment of Acute Myeloid Leukemia.Clin Cancer Res. 2019 Feb 15;25(4):1358-1368. doi: 10.1158/1078-0432.CCR-18-0333. Epub 2018 Jun 29. Clin Cancer Res. 2019. PMID: 29959143
-
Prediction of non-linear pharmacokinetics in humans of an antibody-drug conjugate (ADC) when evaluation of higher doses in animals is limited by tolerability: Case study with an anti-CD33 ADC.MAbs. 2018 Jul;10(5):738-750. doi: 10.1080/19420862.2018.1465160. Epub 2018 May 18. MAbs. 2018. PMID: 29757698 Free PMC article.
-
KK2845, a PBD dimer-containing antibody-drug conjugate targeting TIM-3-expressing AML.Leukemia. 2025 Aug;39(8):1848-1856. doi: 10.1038/s41375-025-02642-2. Epub 2025 May 22. Leukemia. 2025. PMID: 40404985 Free PMC article.
-
Monoclonal antibody therapy in acute myeloid leukemia.Curr Hematol Rep. 2003 Jan;2(1):73-7. Curr Hematol Rep. 2003. PMID: 12901157 Review.
-
The past and future of CD33 as therapeutic target in acute myeloid leukemia.Blood Rev. 2014 Jul;28(4):143-53. doi: 10.1016/j.blre.2014.04.001. Epub 2014 Apr 21. Blood Rev. 2014. PMID: 24809231 Review.
Cited by
-
Targeting CD33 for acute myeloid leukemia therapy.BMC Cancer. 2022 Jan 3;22(1):24. doi: 10.1186/s12885-021-09116-5. BMC Cancer. 2022. PMID: 34980040 Free PMC article.
-
A Phase I dose-escalation study of DCLL9718S, an antibody-drug conjugate targeting C-type lectin-like molecule-1 (CLL-1) in patients with acute myeloid leukemia.Am J Hematol. 2021 May 1;96(5):E175-E179. doi: 10.1002/ajh.26136. Epub 2021 Mar 11. Am J Hematol. 2021. PMID: 33617672 Free PMC article. Clinical Trial. No abstract available.
-
Targeting CLL-1 for acute myeloid leukemia therapy.J Hematol Oncol. 2019 Apr 24;12(1):41. doi: 10.1186/s13045-019-0726-5. J Hematol Oncol. 2019. PMID: 31014360 Free PMC article. Review.
-
Ligand Density Controls C-Type Lectin-Like Molecule-1 Receptor-Specific Uptake of Polymer Nanoparticles.Adv Biosyst. 2020 Nov;4(11):e2000172. doi: 10.1002/adbi.202000172. Epub 2020 Oct 19. Adv Biosyst. 2020. PMID: 33073549 Free PMC article.
-
Translational PK/PD and the first-in-human dose selection of a PD1/IL15: an engineered recombinant targeted cytokine for cancer immunotherapy.Front Pharmacol. 2024 Jun 3;15:1380000. doi: 10.3389/fphar.2024.1380000. eCollection 2024. Front Pharmacol. 2024. PMID: 38887559 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
