A synthetic anti-Frizzled antibody engineered for broadened specificity exhibits enhanced anti-tumor properties
- PMID: 30183492
- PMCID: PMC6284576
- DOI: 10.1080/19420862.2018.1515565
A synthetic anti-Frizzled antibody engineered for broadened specificity exhibits enhanced anti-tumor properties
Abstract
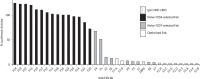
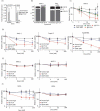
Secreted Wnt ligands play a major role in the development and progression of many cancers by modulating signaling through cell-surface Frizzled receptors (FZDs). In order to achieve maximal effect on Wnt signaling by targeting the cell surface, we developed a synthetic antibody targeting six of the 10 human FZDs. We first identified an anti-FZD antagonist antibody (F2) with a specificity profile matching that of OMP-18R5, a monoclonal antibody that inhibits growth of many cancers by targeting FZD7, FZD1, FZD2, FZD5 and FZD8. We then used combinatorial antibody engineering by phage display to develop a variant antibody F2.A with specificity broadened to include FZD4. We confirmed that F2.A blocked binding of Wnt ligands, but not binding of Norrin, a ligand that also activates FZD4. Importantly, F2.A proved to be much more efficacious than either OMP-18R5 or F2 in inhibiting the growth of multiple RNF43-mutant pancreatic ductal adenocarcinoma cell lines, including patient-derived cells.
Keywords: Wnt signaling; anti-Frizzled synthetic antibodies; pancreatic ductal adenocarcinoma; phage display.
Figures




Similar articles
-
Structure-guided design fine-tunes pharmacokinetics, tolerability, and antitumor profile of multispecific frizzled antibodies.Proc Natl Acad Sci U S A. 2019 Apr 2;116(14):6812-6817. doi: 10.1073/pnas.1817246116. Epub 2019 Mar 20. Proc Natl Acad Sci U S A. 2019. PMID: 30894493 Free PMC article.
-
Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors.Nat Med. 2017 Jan;23(1):60-68. doi: 10.1038/nm.4219. Epub 2016 Nov 21. Nat Med. 2017. PMID: 27869803
-
Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma.Proc Natl Acad Sci U S A. 2013 Jul 30;110(31):12649-54. doi: 10.1073/pnas.1307218110. Epub 2013 Jul 11. Proc Natl Acad Sci U S A. 2013. PMID: 23847203 Free PMC article.
-
Frizzled Receptors as Potential Therapeutic Targets in Human Cancers.Int J Mol Sci. 2018 May 22;19(5):1543. doi: 10.3390/ijms19051543. Int J Mol Sci. 2018. PMID: 29789460 Free PMC article. Review.
-
Progress in the development of modulators targeting Frizzleds.Pharmacol Res. 2024 Aug;206:107286. doi: 10.1016/j.phrs.2024.107286. Epub 2024 Jun 25. Pharmacol Res. 2024. PMID: 38936522 Review.
Cited by
-
Structure-guided design fine-tunes pharmacokinetics, tolerability, and antitumor profile of multispecific frizzled antibodies.Proc Natl Acad Sci U S A. 2019 Apr 2;116(14):6812-6817. doi: 10.1073/pnas.1817246116. Epub 2019 Mar 20. Proc Natl Acad Sci U S A. 2019. PMID: 30894493 Free PMC article.
-
Frizzled receptors (FZDs) in Wnt signaling: potential therapeutic targets for human cancers.Acta Pharmacol Sin. 2024 Aug;45(8):1556-1570. doi: 10.1038/s41401-024-01270-3. Epub 2024 Apr 17. Acta Pharmacol Sin. 2024. PMID: 38632318 Free PMC article. Review.
-
Targeting cancer stem cells in drug discovery: Current state and future perspectives.World J Stem Cells. 2019 Jul 26;11(7):398-420. doi: 10.4252/wjsc.v11.i7.398. World J Stem Cells. 2019. PMID: 31396368 Free PMC article. Review.
-
PIK3C3 Inhibition Promotes Sensitivity to Colon Cancer Therapy by Inhibiting Cancer Stem Cells.Cancers (Basel). 2021 Apr 30;13(9):2168. doi: 10.3390/cancers13092168. Cancers (Basel). 2021. PMID: 33946505 Free PMC article.
-
Tailored tetravalent antibodies potently and specifically activate Wnt/Frizzled pathways in cells, organoids and mice.Elife. 2019 Aug 27;8:e46134. doi: 10.7554/eLife.46134. Elife. 2019. PMID: 31452509 Free PMC article.
References
-
- Hoppler S, Moon RT. Wnt signaling in development and disease molecular mechanisms and biological functions. Hoboken (New Jersey): Wiley Blackwell; 2014. 1 online resource.
-
- Hart MJ, De Los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 8;1998:573–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
