Injury of Adult Zebrafish Expressing Acvr1lQ204D Does Not Result in Heterotopic Ossification
- PMID: 30183553
- PMCID: PMC6277083
- DOI: 10.1089/zeb.2018.1611
Injury of Adult Zebrafish Expressing Acvr1lQ204D Does Not Result in Heterotopic Ossification
Abstract
Fibrodysplasia Ossificans Progressiva (FOP) is a rare, autosomal dominant genetic disorder in humans characterized by the gradual ossification of fibrous tissues, including skeletal muscle, tendons, and ligaments. In humans, mutations in the Type I BMP/TGFβ family member receptor gene, ACVR1, are associated with FOP. Zebrafish acvr1l, previously known as alk8, is the functional ortholog of human ACVR1. We previously created and characterized the first adult zebrafish model for FOP by generating animals harboring heat shock-inducible mCherry-tagged constitutively active Acvr1l (Q204D). Since injury is a known trigger for heterotopic ossification (HO) development in human FOP patients, in this study, we investigated several injury models in Acvr1lQ204D-expressing zebrafish and the subsequent formation of HO. We performed studies of Activin A injection, cardiotoxin (CTX) injection, and caudal fin clip injury. We found that none of these methods resulted in HO formation at the site of injury. However, some of the cardiotoxin-injected and caudal fin-clipped animals did exhibit HO at distant sites, including the body cavity and along the spine. We describe these results in the context of new and exciting reports on FOP, and discuss future studies to better understand the etiology and progression of this disease.
Keywords: activating ACVR1 mutations; fibrodysplasia ossificans progressiva; heterotopic ossification.
Conflict of interest statement
No competing financial interests exist.
Figures
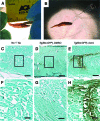


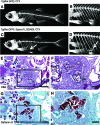



References
-
- Kaplan FS, Tabas JA, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J Bone Joint Surg Am 1993;75:220–230 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

