3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade
- PMID: 30183789
- PMCID: PMC6274590
- DOI: 10.1039/c8lc00322j
3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade
Abstract
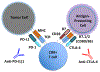
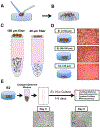
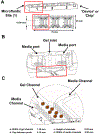
Microfluidic culture has the potential to revolutionize cancer diagnosis and therapy. Indeed, several microdevices are being developed specifically for clinical use to test novel cancer therapeutics. To be effective, these platforms need to replicate the continuous interactions that exist between tumor cells and non-tumor cell elements of the tumor microenvironment through direct cell-cell or cell-matrix contact or by the secretion of signaling factors such as cytokines, chemokines and growth factors. Given the challenges of personalized or precision cancer therapy, especially with the advent of novel immunotherapies, a critical need exists for more sophisticated ex vivo diagnostic systems that recapitulate patient-specific tumor biology with the potential to predict response to immune-based therapies in real-time. Here, we present details of a method to screen for the response of patient tumors to immune checkpoint blockade therapy, first reported in Jenkins et al. Cancer Discovery, 2018, 8, 196-215, with updated evaluation of murine- and patient-derived organotypic tumor spheroids (MDOTS/PDOTS), including evaluation of the requirement for 3D microfluidic culture in MDOTS, demonstration of immune-checkpoint sensitivity of PDOTS, and expanded evaluation of tumor-immune interactions using RNA-sequencing to infer changes in the tumor-immune microenvironment. We also examine some potential improvements to current systems and discuss the challenges in translating such diagnostic assays to the clinic.
Conflict of interest statement
Disclosure of Potential Conflicts
A. Aref, D.A. Barbie, and R.W. Jenkins have ownership interest in pending U.S. Patent Application No. 15/540,346.. R.D. Kamm has ownership interest (including patents) in AIM Biotech, D.A. Barbie is a consultant/advisory board member for N of One.
Figures



References
-
- Mahoney KM, Rennert PD and Freeman GJ, Nat Rev Drug Discov, 2015, 14, 561–584. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

