Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints
- PMID: 30184160
- PMCID: PMC6270990
- DOI: 10.1210/er.2018-00006
Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints
Abstract
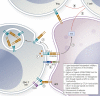
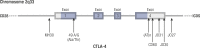
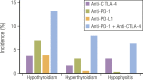
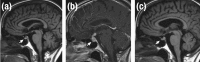
Immune checkpoints are small molecules expressed by immune cells that play critical roles in maintaining immune homeostasis. Targeting the immune checkpoints cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD-1) with inhibitory antibodies has demonstrated effective and durable antitumor activity in subgroups of patients with cancer. The US Food and Drug Administration has approved several immune checkpoint inhibitors (ICPis) for the treatment of a broad spectrum of malignancies. Endocrinopathies have emerged as one of the most common immune-related adverse events (irAEs) of ICPi therapy. Hypophysitis, thyroid dysfunction, insulin-deficient diabetes mellitus, and primary adrenal insufficiency have been reported as irAEs due to ICPi therapy. Hypophysitis is particularly associated with anti-CTLA-4 therapy, whereas thyroid dysfunction is particularly associated with anti-PD-1 therapy. Diabetes mellitus and primary adrenal insufficiency are rare endocrine toxicities associated with ICPi therapy but can be life-threatening if not promptly recognized and treated. Notably, combination anti-CTLA-4 and anti-PD-1 therapy is associated with the highest incidence of ICPi-related endocrinopathies. The precise mechanisms underlying these endocrine irAEs remain to be elucidated. Most ICPi-related endocrinopathies occur within 12 weeks after the initiation of ICPi therapy, but several have been reported to develop several months to years after ICPi initiation. Some ICPi-related endocrinopathies may resolve spontaneously, but others, such as central adrenal insufficiency and primary hypothyroidism, appear to be persistent in most cases. The mainstay of management of ICPi-related endocrinopathies is hormone replacement and symptom control. Further studies are needed to determine (i) whether high-dose corticosteroids in the treatment of ICPi-related endocrinopathies preserves endocrine function (especially in hypophysitis), and (ii) whether the development of ICPi-related endocrinopathies correlates with tumor response to ICPi therapy.
Figures


References
-
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. - PMC - PubMed
-
- Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee . Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–iv142. - PubMed
-
- Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, Golstein P. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328(6127):267–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

